The prime responsibility of blood transfusion services in India is to provide safe blood. The donated blood is tested for human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), malaria and syphilis. In India, the screening of donated blood for syphilis is performed by rapid plasma reagin (RPR) or venereal disease research laboratory (VDRL), whereas the World Health Organization (WHO) recommends screening of syphilis in blood donors by enzyme-linked immunosorbent assay (ELISA). Therefore, the aim of this study was to evaluate the performance of RPR and ELISA with the Treponema pallidum hemagglutination assay (TPHA – the gold standard) for the detection of syphilis in blood donors.
MethodsIn this cross-sectional study, 1524 consecutive whole blood donors were screened from April to October 2022. All blood samples collected during the study period were tested by RPR, ELISA and the TPHA and the results obtained were compared.
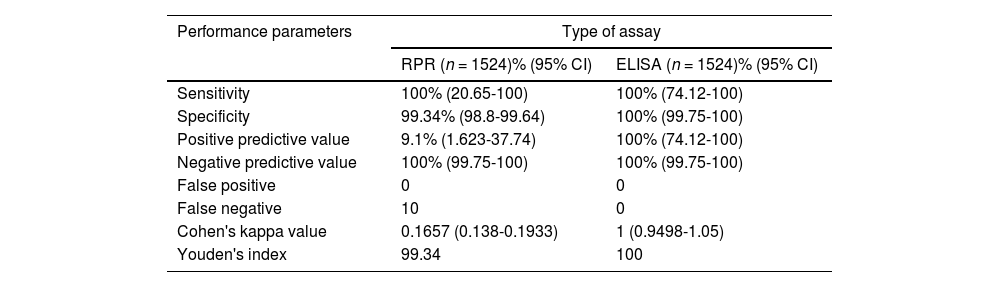
ResultsThe seroprevalence of syphilis in blood donors in this study was 0.06% by RPR and 0.72% by ELISA and TPHA. On considering ELISA and the TPHA as the gold standard, ELISA had comparable sensitivity (100%), a higher specificity (100% vs. 99.34%), a higher positive predictive value (PPV - 100% vs. 9.1%) and no biological false positive/false negative results (0 vs. 10 false negatives) when compared to RPR.
ConclusionELISA performed better as a screening assay than RPR in the detection of syphilis in blood donors, which is in agreement with the WHO recommendations for syphilis testing in blood donors with low prevalence.
Syphilis is a sexually transmitted infection caused by spirochete Treponema pallidum.1,2 The other infection transmission routes are in utero and rarely, blood transfusion. Syphilis still remains one of the most significant health problems worldwide, thus, accurate diagnosis is of utmost importance for disease control and patient management.1-4
During infections, two types of antibodies are produced: treponemal and non-treponemal (NT). Amongst the assays available, non-treponemal antibodies (cardiolipin, reagin or lipoidal) are detected by rapid plasma reagin (RPR) or venereal disease research laboratory (VDRL), whereas treponemal antibodies are detected by the T. pallidum hemagglutination assay (TPHA), enzyme-linked immunosorbent assay (ELISA) and fluorescent treponemal antibody-absorption (FTA-abs) tests.4-6 Non-treponemal tests are most commonly used and are preferred over treponemal tests for various reasons such as ease of use, low cost, less technical expertise required, no sophisticated equipment requirement and high sensitivity in the detection of active infections.6,7 However, the limitations of the non-treponemal tests are lower specificity and more false negative (prozone phenomena)/false positive results.3-5
The prime objective of blood transfusion services in India is to provide safe blood. This is possible due to the collection of blood from voluntary non-remunerated donors, pre-donation screening and testing for transfusion transmitted infections (TTI). In India, it is mandatory to test donated blood serologically for human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), malaria and syphilis.3,5,8 Currently, nucleic acid testing (NAT) and hepatitis B core antibody (anti-HBc) are recommended but not mandatory screening assays, therefore, few blood centres across India are performing these tests.5,8
Syphilis was one of the first TTI to be tested in blood centres across the world with the first reported case of transfusion-transmitted syphilis being reported in 1915.3 As per The Drugs and Cosmetics Act and Rules 1945, the screening of donated blood for syphilis by RPR or VDRL is mandatory in India8, whereas the World Health Organization (WHO) recommends screening for syphilis in blood donors by ELISA.3-5 Recent studies show that ELISA has better sensitivity and specificity, hence, a better positive predictive value (PPV) and less biological false positivity (BFP) in comparison to RPR.1,5-7 The choice of the method to be used to detect syphilis is determined by each blood centre. Thus, there is discordance in the screening methodology utilized for syphilis screening between Indian blood centres and the WHO recommended screening strategy with ELISA. Hence, this study aimed to evaluate the performance of RPR and automated ELISA compared to TPHA (gold standard) to detect syphilis in blood donors.
Materials and methodsThis cross-sectional study was conducted in the Department of Immunohematology and Blood Transfusion, Bharati Vidyapeeth Medical College and Hospital from April 2022 to October 2022 after Institutional Ethics Committee approval. A total of 1524 consecutive healthy, eligible whole blood donors providing informed consent were included in the study. Deferred/rejected blood donors and lipemic/hemolyzed samples were excluded.
During blood donation, blood samples were collected in two pilot tubes (3 mL in ethylenediaminetetraacetic acid - EDTA; 4 mL in clot accelerator vacutainer) for serology and TTI testing. All consecutive blood donor samples were tested by RPR (Carbogen®, Tulip Diagnostics P Ltd., India), ELISA using a Bio-Rad Evolis fully automated processor (syphilis total Ab, Bio-Rad, Marnes-la-Coquette, France) and the TPHA (Bio-Rad, Marnes-la-Coquette, France) to detect syphilis in blood donors. TPHA was considered the gold standard for the study. All tests were performed as per the manufacturer's instructions.
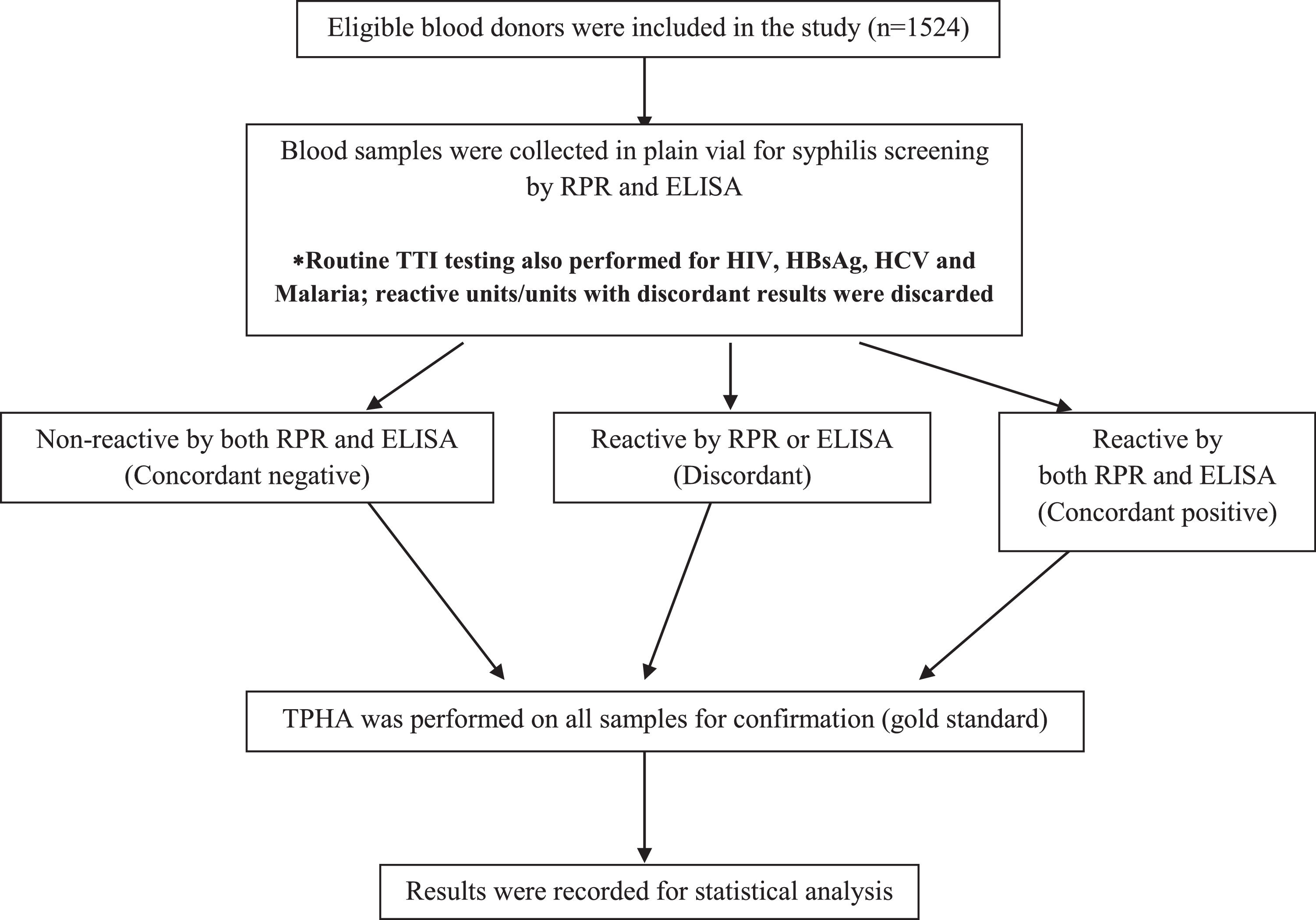
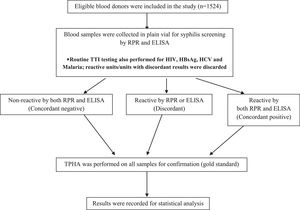
All tests were performed prior to the release of blood components into the stock inventory. In cases of non-reactivity, the blood components were added to the inventory, while in cases of reactive and/or discordant results (non-agreement between RPR, ELISA and TPHA) blood components were labelled as unfit for transfusions and discarded. Figure 1 summarizes the study algorithm.
The data was entered into an Excel spreadsheet and analysis was performed using IBM SPSS statistical software (version 25). A comparative performance evaluation was carried out for the following parameters: sensitivity, specificity, PPV, negative predictive value (NPV) and Youden's index with 95% confidence limits. Cohen's kappa test was also performed to determine the agreement between the screening assays. A p-value <0.05 was considered to be statistically significant.
ResultsA total of 1524 (1427 males: 93.6%; 86 females: 6.4%) consecutive whole blood donors were screened for syphilis by RPR, ELISA and TPHA during the study period. The average age was 31.6 ± 9.6 years. Eleven donor samples were found to be reactive for syphilis either by RPR, ELISA or TPHA. Seroprevalence of syphilis was found to be 0.06% by RPR and 0.72% by ELISA and TPHA. None of these samples were found to be reactive for malaria, HBV, HCV or HIV.
Comparison of rapid plasma reagin and enzyme-linked immunosorbent assay against Treponema pallidum hemagglutination assay (gold standard)
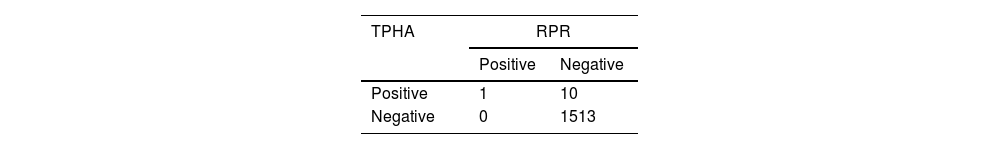
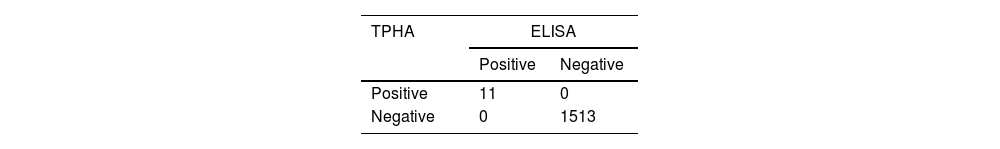
Tables 1 and 2 summarize the serostatus of the donor samples tested for syphilis by RPR and ELISA against TPHA. The analytical sensitivity of RPR for syphilis detection was 100% with 99.34% specificity, while ELISA showed 100% sensitivity and specificity (Table 3). The PPV of RPR (9.1%) was found to be low in comparison to ELISA (100%; Table 3). Perfect agreement was noted between ELISA and TPHA (к = 1), while RPR and TPHA showed only slight agreement (к = 0.1657).
Comparative analysis of rapid plasma regain (RPR) and enzyme-linked immunosorbent assay (ELISA).
CI: Confidence interval
Various types of screening assays are available for blood donor screening for TTIs. The use of a particular screening assay is determined by country-specific guidelines issued by the policy makers/government as well as the performance characteristics (sensitivity, specificity, PPV, NPV and Youden's index). Thus, transfusion services use different permutations and combinations of screening assays for TTI testing in blood donors with preference given to high sensitivity (∼100%) with acceptable specificity and cost-effectiveness.1,3-5 Wastage of blood components due to false positive results is still considered acceptable in comparison to false negative results which compromise the blood and patient safety.9-11
However, no single ideal screening assay for syphilis detection in blood donors is available as each assay comes with its own limitations in terms of cost-effectiveness, ease of use, need of technical expertise, equipment, performance parameters (sensitivity, specificity) and the results vary according to the stage of syphilis infection.5,9,10 The aim of the present study was to evaluate the performance of RPR and ELISA against TPHA as a gold standard for the detection of syphilis in blood donors.
The sensitivity of RPR (100%) was found to be comparable to ELISA (100%) in this study, while the specificity of ELISA was 100% and RPR was 99.34%. Similar results have been reported by Sachdev et al.5, Negash et al.1, Saral et al.9, Naidu et al.10 reported that RPR has 0% specificity which should be interpreted with caution as these tests were performed in an apparently healthy blood donor population. Apart from this, the PPV of ELISA (100%) was also found to be higher than that of RPR (9.1%) in the present study which is similar to the results in the published literature.1,3-5,9-11
In the present study, RPR showed a low PPV and consequently a high false negative rate, despite high sensitivity, thus, demonstrating its poor performance as a screening assay for syphilis detection. The various reasons for false negative results are low specificity, technical errors (insufficient distribution of antigens in a sample not previously placed on the entire test area surface), reagent temperature and the prozone phenomena noted in individuals with early primary syphilis.5,9-11 To rule out false negative results due to the prozone phenomena, blood samples should be re-tested after serial dilution of the serum/plasma which will clearly demonstrate a positive result as the antigen-antibody ratio reaches the optimal range.11 However, this was not performed in the current study; thus, it would be difficult to comment whether these samples represent actual true negative/positive results. On the contrary, the published literature reports more BFP with RPR.1,3-5,9,10 Though, we did not observe any false positive results with RPR and ELISA. BFP is noted due to cross-reactivity seen with other molecules in various other conditions such as viral infections, pregnancy, malignant neoplasms, autoimmune disorders, systemic lupus erythematosus, thyroiditis, rheumatoid arthritis, atopic dermatitis, vaccination and advanced age.5,11 The reason for no BFP seen in the current study could be attributable to testing a small number of donor samples.
This study demonstrates that ELISA, as a screening assay, performed better than RPR in terms of performance characteristics. Non-treponemal tests like RPR usually become non-reactive after successful treatment. Therefore, they are more useful to monitor the response to treatment.5 Around 8-10% of syphilis patients with various stages of infection are known to give false negative results in RPR/VDRL tests due to the prozone phenomena, therefore, the use of such tests alone as a screening assay can be detrimental. Hence, screening donated blood with treponemal tests, which are known to have high sensitivity and specificity, is a more practical option rather than carrying out serum dilutions.12 The several other advantages of using ELISA as a screening assay for the detection of syphilis are automation, higher throughput, easier record keeping, traceability of results/reagents, use of a single pilot tube for testing other TTI markers and ensuring compliance to good manufacturing practices. However, the major concerns with the implementation of treponemal tests such as ELISA as a screening assay is the high BFP rates which can result in higher discard rates, thus, affecting the blood donor pool.4,5,7,10-12
The limitations of this study were (i) small sample size, (ii) single centre study, and (iii) testing performed on samples from healthy blood donors, who are known to have a very low prevalence as they represent the healthy general population.
To conclude, the seroprevalence of syphilis in blood donors in this study was 0.06% by RPR and 0.72% by ELISA and TPHA. Despite the comparable sensitivity (100%) shown by RPR, ELISA performed better with higher specificity (100% vs. 99.34%), higher PPV (100% vs. 9.1%) and no BFP/false negative results (0 vs. 10 false negative) when compared to RPR. Therefore, the results of present study support the WHO recommendation3-5 of using ELISA as a screening assay for syphilis detection in blood donor populations with low prevalence. However, there is a need to evaluate the performance of ELISA as a screening assay on a large scale with a blood donor population representing different geographical settings in Indian transfusion services.