Acute promyelocytic leukemia (APL), is a clinically well-defined subtype of acute myeloid leukemia (AML), which is now considered curable. The advent of all-trans-retinoid-acid (ATRA) resulted in the patients achieving better overall survival. It is presumed that secondary malignancies result from the exposure to alkylating agents or DNA topoisomerase II inhibitors.1 We describe a case of a 30-year-old male who presented to us for the first time in 2013 and diagnosed with APL. He achieved complete clinical and molecular remission after treatment with an anthracycline and ATRA combined. After remaining disease free for about 6 years, he again presented to us with ankle pain associated with hyperleucocytosis. During the hospital stay, his complete workup revealed the diagnosis of Chronic Myeloid Leukemia (CML) without any evidence of APL relapse. Although therapy related acute myeloid leukemia and myelodysplastic syndrome have been reported as secondary malignancies in APL but chronic myeloid leukemia is very rarely reported.2 We describe a case of treated APL that developed CML as a secondary malignancy in the light of previous medical literature.
BackgroundChronic Myeloid Leukemia developing as a secondary malignancy after treated acute promyelocytic leukemia is a very rare phenomenon. Here, we describe hyperleucocytosis associated with bilateral ankle pain in a 30-year-male who was successfully treated for APL 6 years back. He was finally diagnosed as CML in the recent admission. The patient was vitally stable on presentation with initial suspicion of APL relapse due to high white cell count, however complete workup including bone marrow trephine, cytogenetics and nested RT-PCR revealed the diagnosis of CML in chronic phase. In a successfully treated patient with APL, a physician should be aware of the possibility of the development of CML. Purpose of writing the case report is to highlight the rare possibility of CML occurring in treated APL patients as a secondary malignancy.
Case presentationIn 2013, a 30-year-old male presented to emergency department with history of undocumented fever, non-productive cough and right arm swelling since 1 week. Initial laboratory investigations showed Hemoglobin (Hb): 11.7 g/dL; Hematocrit (Hct): 344; Mean cell volume (MCV): 52 fL; Mean cell hemoglobin (MCH): 17.7 pg; WBC: 147.6 × 109/L; Absolute neutrophil count (ANC): 35.4 × 109/L; Blast: 72%; Platelets: 36 × 109/L. While the rest of the baseline labs including renal function tests, liver function tests and electrolyte were normal, there was an increase in the levels of uric acid: 8.6 mg/dl and LDH: 1703 IU/L. Ultrasound Doppler right arm showed evidence of thrombosis in brachial vein. He was admitted under hematology-oncology service. PT/APTT was normal while D-Dimer levels were 80 mg/L FEU and fibrin levels 272 mg/dl. Bone marrow flowcytometry showed bright reactivity to myeloid markers including myeloid peroxidase (MPO) and negativity to HLA-DR. Bone marrow aspirate showed 91% blast cells with abnormal promyelocytes. Bone trephine showed diffuse infiltration with blast cells and bone marrow cytogenetics was normal. PML/RARA translocation t (15; 17) was detected using both FISH (Fig. 1) and real-time RT-PCR technique. AML inversion 16, translocation t (8; 21), nucleopshosmin 1 (NPM1) and; FLT3 ITD and 835 mutations were not detected. A diagnosis of high risk APL was made and patient started on induction therapy. Induction was given with 7+3 regimen including Cytarabine at 100 mg/m2 intravenous (IV) from day 1 to day 7, with Daunorubicin at 45 mg/m2 IV. from day 1 to day 3, in addition to ATRA at 40 mg/m2 in two divided doses. Post induction marrow showed complete remission and PML-RARA t (15; 17) was not detected by real-time RT-PCR. Induction was followed by four cycles of consolidation with ATRA, anthracycline and mitoxantrone. Post-consolidation bone marrow was in remission and PML-RARA t (15; 17) was not detected by real-time RT-PCR.
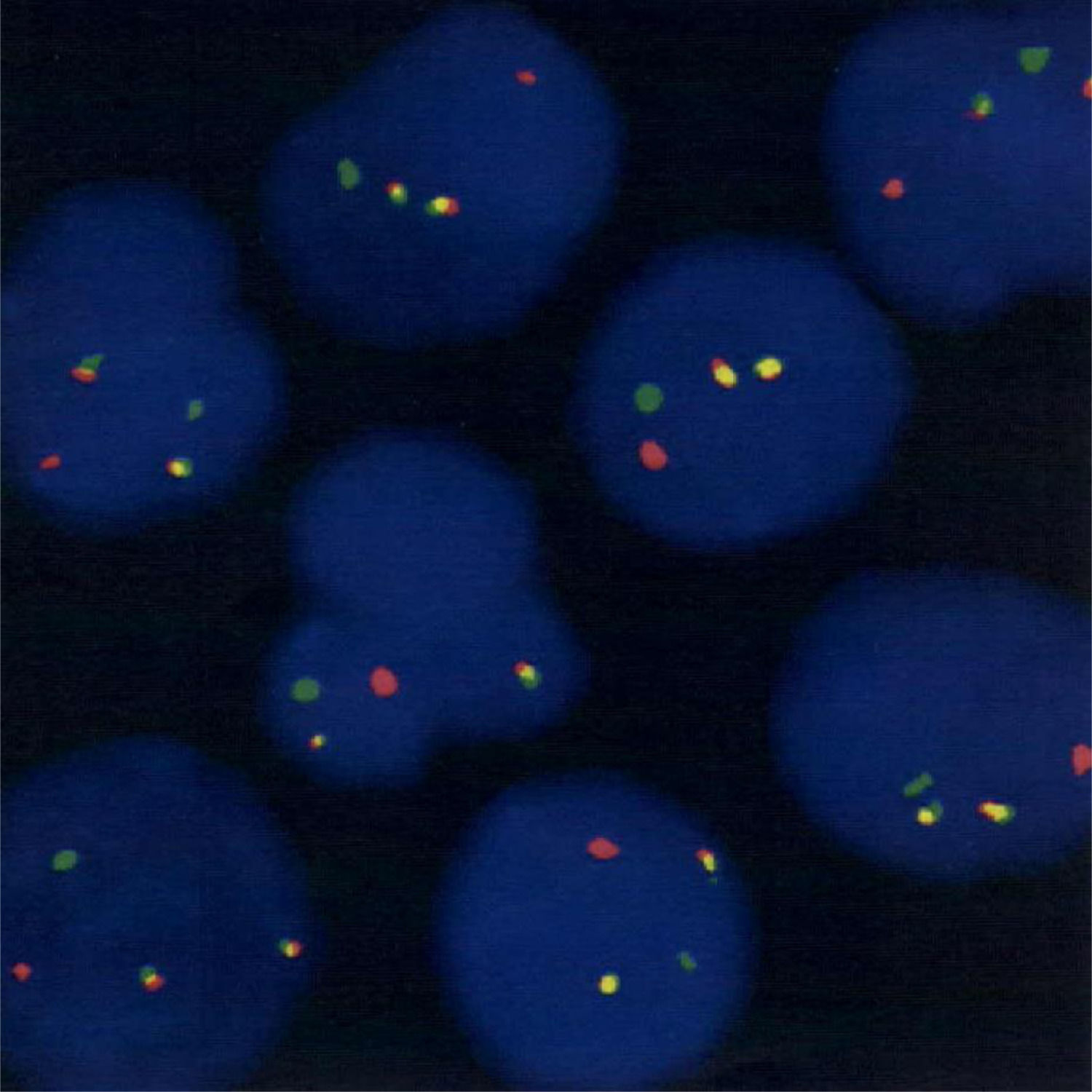
FISH image (orange signal: 15q22 LSI PML, green signals; 17q21.1 LSI RARA). Result: t(15;17)(q22;q21) is detected in 95% of the 500 nuclei counted. Method: Dual colour translocation probe is used for the detection of PML/RARA gene fusion in patient blood sample. Interpretation: In a normal cell two orange signals of PML and two green signals of RARA representing normal copies, whereas fusion of PML/RARA will display one orange, one green and one orange/green (yellow) fusion signal pattern.
The patient remained in complete remission until January 2020 when he presented to us at clinic with incidental high white cell counts (TLC 50 × 109) from an outside laboratory with concerns of bilateral ankle pain. With a suspicion of disease relapse, diagnostic workup was carried out.
InvestigationsInitial laboratory investigations showed Hb 13.5 g/dl, TLC 39.7 × 109 /L with absolute neutrophil count (ANC) 31.7 × 109/L and platelets: 315 × 109/L. Peripheral film showed leukocytosis with myelocytes, metamyelocytes and leucoerythroblastic blood picture. No blast cells were observed. Blood chemistry showed uric acid 10.4 mg/dl. Rest of the baseline laboratory investigations including renal function, liver function tests and electrolytes were within normal range. Antinuclear antibody (ANA), anti-DS DNA and anti-cyclic citrullinated peptide (anti-CCP) were negative. PML/RARA translocation t (15; 17) was not detected by real-time RT-PCR technique. X-ray of bilateral ankles was negative for any pathology.
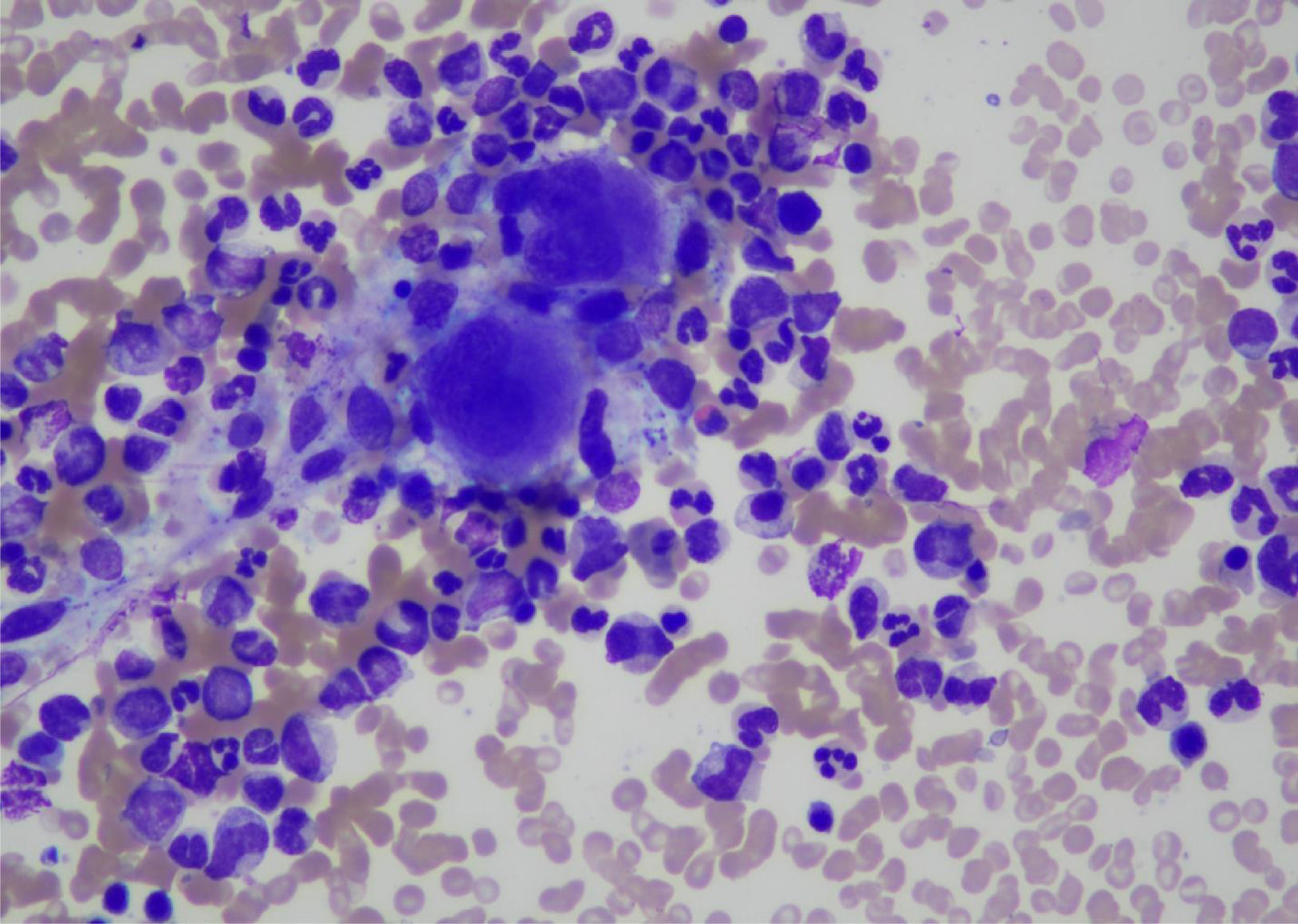
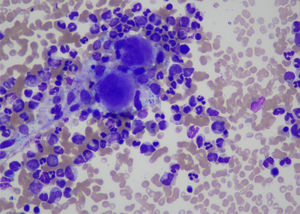
Bone marrow biopsy was performed which was consistent with the diagnosis of CML (Chronic phase). Bone marrow aspirate was a hyper cellular specimen showing trilineage hematopoiesis with myeloid hyperplasia consisting of immature granulocytic precursors such as promyelocytes, myelocytes and metamyelocytes. Erythropoiesis was relatively suppressed. Myeloid: Erythroid ratio was 32:1. (Fig. 2)
Bone trephine showed cellularity of around 90–95%. Cellular areas showed myeloid hyperplasia, with adequate megakaryocytes and relatively suppressed erythropoiesis. Overall bone marrow findings were consistent with myeloproliferative disorder (chronic myeloid leukemia in chronic phase).
BCR ABL1 p210 (b3a2) transcript was detected by nested RT-PCR Qualitative which was estimated to represent 21% IS (International scale) by real-time RT-PCR Quantitative. Bone marrow cytogenetics showed 46, XY, t (9; 22) (q34; q11.2), that is 20 cells were counted and all cells were positive for Philadelphia chromosome. (Fig. 3)
TreatmentPatient was admitted to general ward and managed symptomatically for pain in addition to baseline laboratory work-up. Rheumatology service was involved for bilateral ankle pain, which suggested conservative management. High white cell counts settled following treatment with hydroxyurea. Final diagnosis was CML Chronic phase, according to WHO. 2016 classification. Patient was started on nilotinib 600 mg per oral, once a day and discharged for later follow-up as outpatient.
Outcome and follow-upPatient was followed up at clinic after discharge. He achieved a hematological response at 4 weeks and achieved early molecular response at 3 months based on BCR-ABL Quantification by PCR (BCR-ABL 4%). He is currently on same dose of nilotinib. His last cbc shows Hb 12.8 g/dl, TLC 10.0 × 109 /L with 55% neutrophils, 36% lymphocytes and platelets 219 × 109 /L. He has been under blood count surveillance with a plan to continue nilotinib and monitor for molecular response at three months.
DiscussionAs APL has a very good response to treatment and patients have increased long-term survival, therapy related leukemia has become a foremost concern. There are few reports of therapy related myelodysplastic syndrome (t-MDS) and acute myeloid leukemia (t-AML) in treated patients of APL.2,3
There is also an increasing trend in cases of therapy-related CML (t-CML) that arise in patients treated for primary hematological disorders like Hodgkin and non-Hodgkin lymphoma, acute leukemia and chronic lymphocytic leukemia in addition to solid tumors like ovarian, breast and uterine cervical cancers.4,5 32 cases of treatment related CML were studied and it was suggested that there was no clinically significant difference between treatment related CML and the de novo CML cases.6
In previous medical literature, therapy related myeloid neoplasms have been reported, in patients who were treated with chemotherapy for APL. However only two documented cases of transformation to CML were found. In the first case, Sakamaki et al. reported a 62-year-old male who was diagnosed and treated for APL in 1978.7 He achieved complete remission. However, he had relapse of the disease in February 1981. He was then treated with cytosine arabinoside, aclarubicin, 6MP and prednisolone. He achieved remission post treatment and remained disease free for next 5 years after which he presented with leukocytosis and was diagnosed as case of CML with Philadelphia translocation t (9:22) positive in September 1986. His leukocytosis was controlled with the use of busulfan.
Similarly Huang et al. reported a 43-year-old male in 2001 who was diagnosed with APL and treated with ATRA and anthracycline followed by maintenance of 2 years and achieved complete remission.8 After 7 years of disease free interval, he came back with complains of leukocytosis and fatigue and diagnosed as Philadelphia positive CML. Imatinib was prescribed but was not taken due to financial reasons. After achieving a temporary hematological response at 1 month with hydroxyurea, interferon- α and arsenic trioxide (ATO), his disease progressed rapidly to accelerated phase. He became non-compliant to treatment and died after 2 months with sudden gastrointestinal hemorrhage.
Currently studies are also being conducted on the effect of ATO, which is now used as first line in low risk APL patients. Norsworthy et al. in a SEER Medicare analysis reported a high incidence of second malignancies in APL patients treated with ATO, although the risk was similar to patients who received other APL therapies, which were mostly solid tumors.9 However further prospective research into second malignancies following ATO is warranted.
Although it is clear that the benefits of therapy outweigh the long-term side effects, this case shows the importance of careful long-term monitoring of patients treated for APL.


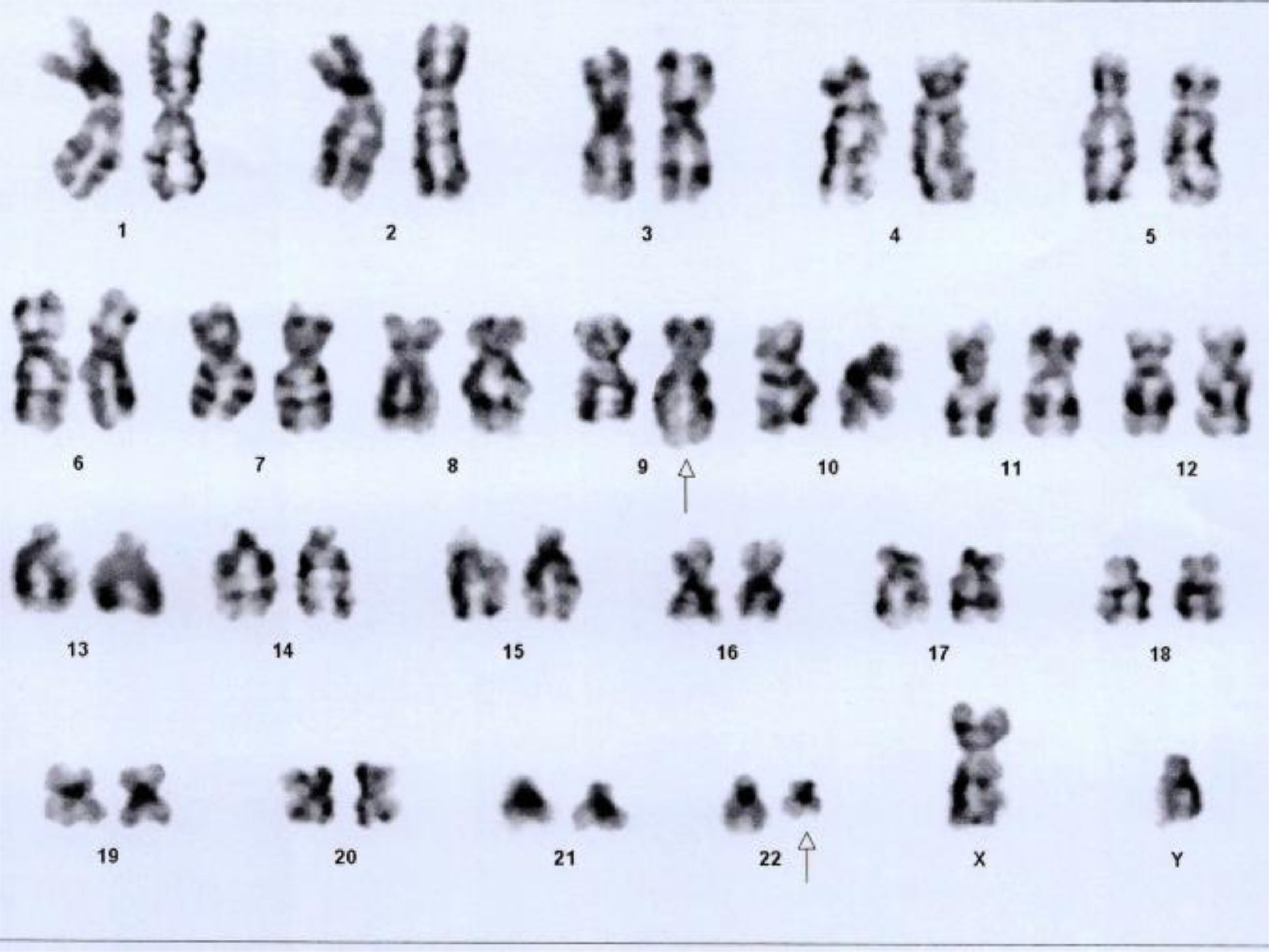
![Bone marrow cytogenetics showing translocation between chromosomes 9q34 and 22q11.2, resulting in Philadelphia chromosome. Number of cells counted: 20; Number of cells analyzed: 20; Number of cells karyogramed: 12; Banding methods: GTG Banding. Result: 46,XY,t(9;22)(q34;q11.2)[20]. Bone marrow cytogenetics showing translocation between chromosomes 9q34 and 22q11.2, resulting in Philadelphia chromosome. Number of cells counted: 20; Number of cells analyzed: 20; Number of cells karyogramed: 12; Banding methods: GTG Banding. Result: 46,XY,t(9;22)(q34;q11.2)[20].](https://static.elsevier.es/multimedia/25311379/0000004400000004/v2_202211230703/S2531137921000730/v2_202211230703/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w93OM6WmS6o9DeZl+SVh74uo=)



