Sickle cell disease is the most widely recognized hemoglobinopathy affecting about 20 million people worldwide.1 However, there had been no cure for this genetic disorder until about two decades ago when the first bone marrow stem cell transplant was carried out.1 The reported cure rate was 85% and dramatically increased to 95% by adding ATG to the conditioning regimen.2,3
ATG is polyclonal gamma immunoglobulin derived from the serum of rabbits or horses injected with human thymus lymphocytes. It is primarily used as a lymphocyte-selective immunosuppressant to prevent the development of graft versus host disease (GvHD) after hematopoietic stem cell transplantation (HSCT) and prevent or treat acute cellular rejection after solid organ transplantation. Additionally, it is the first-line therapy recommended for severe aplastic anemia in the absence of a sibling donor.4,5
The most commonly reported side effects of ATG are fever, chills, leukopenia, lymphopenia, thrombocytopenia, allergic reaction, serum sickness and rarely anaphylaxis,5,6 while the cardiovascular adverse reactions include myocarditis, cardiac irregularity, chest pain, hypertension, hypotension, tachycardia, and bradycardia (6)
Loushin et al. recently reported a 60-year-old male with a delayed onset cardiopulmonary reaction, including bradycardia, after ATG infusion.7 Another case series described significant sinus bradycardia in a 63-year-old female on the third day of ATG treatment.8
Regardless of these reports, bradycardia in children during treatment with ATG has not been reported frequently and is not well recognized by the physician. We are reporting on a child with sickle cell disease, without any pre-existing cardiovascular disease or arrhythmias, who underwent immunosuppressive therapy with ATG, along with methylprednisolone as preparation for HSCT, and experienced profound asymptomatic sinus bradycardia that subsided after stopping ATG.
Case reportA 9-year-old boy who is known to have sickle cell disease (SCD) was scheduled for allogeneic HSCT from his brother, which was indicated based on his clinical presentation with recurrent stroke, splenic sequestration, and pain crises despite medical management. He was evaluated thoroughly prior to HSCT with brain magnetic resonance imaging, pulmonary function tests, and thyroid function, which have been reported as normal. His heart rate in previous visits was documented to range between 70-110 beats/min, and his echocardiography revealed normal structure with ejection fraction (EF) of 63% (normal EF ≥ 55)9,10 and fractional shortening (FS) of 34% (normal FS 26-45%).9,10 A week before the presentation, the patient underwent an exchange transfusion, and his HBSS had been reduced to <30%.
The patient was admitted to the BMT ward to prepare him for HSCT; his physical examination was normal apart from pallor and splenomegaly. Heart Rate 73–89 beat/min, Blood pressure 93–114/54−60, respiratory rate 20–25/min, O2 saturation >95% in room air. Routine laboratory evaluation showed HB 8.4 gm/dl, WBCs 5 × 10^9 /l, platelets 129 × 10^9. The results of biochemistry tests were normal, including the renal function tests, liver function tests, and bone panel as well as TSH and free T4. The treating team started him on the myeloablative-conditioning regimen that includes; rabbit ATG (2.5 mg/kg once daily for four days infused over 14−16 hours), methylprednisolone (1 mg /kg /dose given throughout the infusion of ATG then twice daily), cyclophosphamide (50 mg /kg/day for four days), and busulfan (0.8–1.2 mg/kg/dose for 16 doses), based on current practice regimens. Prior to each ATG infusion, the patient also received premedication with acetaminophen, diphenhydramine, and meperidine 1 mg/kg/dose IV PRN Q6H in case the patient develops rigors.
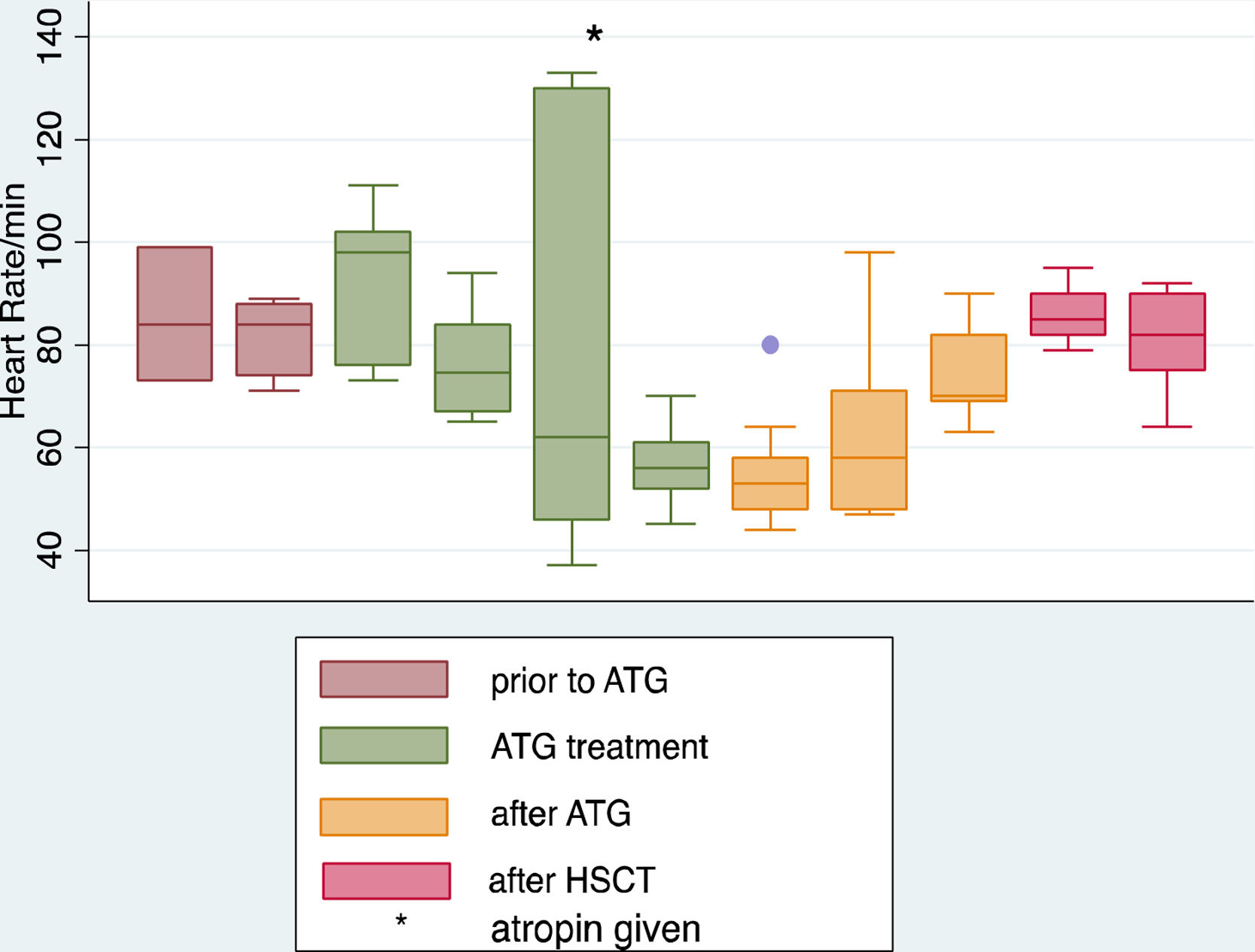
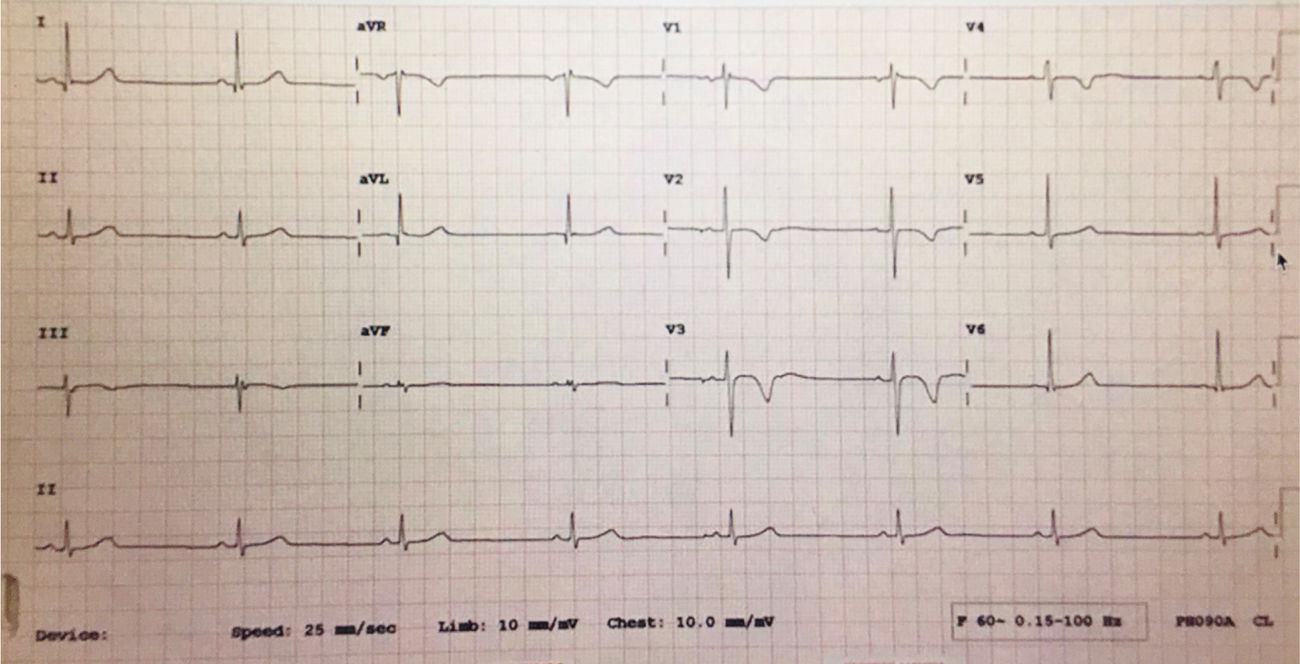
After infusion of the third dose of ATG, the patient developed severe bradycardia, with a heart rate (HR) as low as 40 beats/min, while maintaining normal blood pressure (BP) ranging between 100–115/56−76 and normal perfusion with no other symptoms. The patient immediately shifted to the pediatric critical care unit (PICU) for further monitoring. He remained fully conscious and stable hemodynamically with BP115/75 and normal perfusion, but his heart rate dropped further to 37 beats/min with sinus rhythm on the monitor and 12 lead ECG, the treating intensivist decided to treat him according to the pediatric advanced life support algorithm with one dose of atropine (0.5 mg).11 He responded very well initially where the HR increased to 100 beats/min, and 12 lead ECG revealed sinus bradycardia with a QTc interval of 0.42 s. All serum electrolytes, including potassium, magnesium, and ionized calcium, had been reported within the normal range as well as the echocardiogram, which was done on the second day and showed a normal function with an EF 71%(normal EF ≥ 55) and an FS 41%(normal FS 26–45%).9,10 Holter monitoring for 48 h was done as well to look for any hidden arrhythmia and reported by a pediatric cardiologist as frequent sinus bradycardia with no other arrhythmia. The patient completed the 4th dose of ATG in PICU, and the heart rate continued to range from 45 to 70 beats/min with sinus rhythm and normal BP. In the subsequent days, the patient’s heart rate remained as low as 43 beats/min for two days after ATG and improved to 70–90 beats/min in the third day. Stem cell transplantation was done two days after ATG infusion, and the patient received (acetaminophen, diphenhydramine, and hydrocortisone) 10 days after the last ATG dose, which was tolerated very well by the patient with no complication. Methotrexate and cyclosporine A had been started concomitantly with stem cell infusion day as GVHD prophylaxis and continued according to the protocol. The patient heart rate was stable during and after stem cell transfusion ranging from 80 to 110 beats/min, and then the patient was transferred back to the regular ward. (Figures 1 and 2).
Given the pharmacokinetics of ATG where its onset of action started within 24-hs of infusion and half-life elimination of 2–3 days, our case demonstrates that ATG therapy is probably associated with a definite risk of bradycardia.12 The profound sinus bradycardia that has been experienced by our patient was progressive with the subsequent ATG infusion doses. Additionally, the event seemed to become less severe 2–3 days after the last dose of ATG. This sequential relationship between ATG administration and the incidents of profound bradycardia postulates the most convincing evidence of a causal relationship. In contrast, Godown et al. revealed opposing findings where the bradycardia improved with subsequent ATG doses.13
In the setting that different drugs being administered in conjunction with ATG infusion, it is difficult to attribute the cause of bradycardia to ATG infusion alone. It has been reported that methylprednisolone can cause sinus bradycardia both in adults and children.14,15 We can argue that the bradycardia in our patient is less likely attributed to receiving steroid; IV methylprednisolone was given every 12 h during the ATG treatment course, and the bradycardia developed only in conjunction with ATG administration. Additionally, our patient continued to receive steroids after stem cell transplantation in the form of dexamethasone and hydrocortisone, and no bradycardia had been recorded during this period. Another postulated theory is that the bradycardia effect of steroid therapy may be mediated by elevated intracranial pressure (ICP), where blood pressures usually increased in these patients in conjunction with bradycardia.16 Our patient’s data does not support this possibility, as no symptoms or signs of high ICP were observed during the therapy.
Meperidine was also thought to possibly contribute to the bradycardia. For this reason, it was withdrawn early in the immunosuppressive course, and the last dose was administered just prior to the third ATG infusion. The persistence of bradycardia after discontinuation of meperidine makes it less likely to be the cause.
We believed that other medications (busulfan, cyclophosphamide) were unlikely to be contributory, and no temporal correlation could be demonstrated between any other medication and the bradycardic episodes.
An additional factor to be considered is the timing of the ATG infusions, which was completed while the patient was sleeping. Although some might hypothesize that the sleep state may have contributed to the worsening of bradycardia, more significant bradycardia was noted during ATG infusions than during holter monitoring throughout sleep and while awake after completion of immunotherapy. This suggests that there was a real correlation between the patient’s bradycardia and ATG administration.
The fact that the patient’s HR was documented to be within the normal range in the previous visits along with echocardiogram report that confirmed normal heart structure and function prior to ATG infusion is against the possibility of cardiovascular complication secondary to Sickle cell disease. This is opposite to what Martins et al. reported on cardiovascular autonomic dysfunction (CAD) in patients with SCD, characterized by the reduction of baroreflex sensitivity and HR modulation, resulting in less reflex bradycardia.17
Another possible mechanism that might be contributing to bradycardia is a systemic hemodynamic response to elevated intracranial pressure. However, the patient never experienced any alteration in blood pressures before or after ATG administration, nor any sign or symptom of elevated intracranial pressure.
No significant respiratory sinus arrhythmia or low blood pressure was observed in association with bradycardia, which should have guided our attention to the exaggerated vagal activity. Since the sinus bradycardia developed in an isolated fashion, this manifestation is probably a definite cardiac adverse event.
ATG has been in use for more than 30 years; therefore, its side effect profile is thought to be well described.18,19 Although cardiac adverse events, namely bradycardia, have, to our knowledge, only been reported as a rare side effect of ATG in the manufacturer's drug package insert and in a few published adult case reports, we suspect that bradycardia might be a more critical adverse event of ATG than previously thought.
ConclusionEven though the condition is uncommon but considered as a serious side effect, our case proposes that ATG infusion might be associated with transient profound asymptomatic bradycardia; hence close monitoring of heart rate is crucial during and after ATG treatment. This relationship might be particularly valid for patients with pre-existing cardiovascular disease or receiving any medications with a negative chronotropic effect. Further studies of large cohorts of patients with various underlying illnesses and treated with ATG will be needed to address the cardiac toxicity issue and determine the exact effect of ATG therapy on cardiac function.
Conflict of interestThe authors declare no conflicts of interest.