Acute promyelocytic leukemia (APL), which accounts for about 8% of all acute myeloid leukemias, is characterized by a translocation between chromosomes 15 and 17. This translocation involves the retinoic acid receptor alpha (RARA) gene and the promyelocytic leukemia (PML) gene resulting in the PML-RARA fusion.
One consequence of PML-RARA fusion is a blockage of leukemic cells differentiation that are arrested at the promyelocyte stage. Thus, PML-RARA fusion renders APL sensible to agents that can induce cell differentiation, such as all-trans retinoid acid (ATRA).
The introduction of ATRA changed APL treatment. Once considered the most catastrophic leukemia subtype APL is now a highly curable disease. Treatments combinations using ATRA has been associated with 80–95% remission rates.
Despite its unquestionable role in APL management, ATRA can cause important side effects. Differentiation syndrome, a relatively common ATRA complication, can induce hypotension, dyspnea, fever and can even be lethal. Other ATRA side effects include genital ulcers and vasculitis.
Here we present a rare but important complication of ATRA therapy. A 36-year-old male patient that developed myositis during his frontline therapy for APL.
Case presentationA 36-year-old male, with no significant medical history aside from nephrolithiasis, presented headaches fatigue, petechial rash and hematomas for the past 30 days and was admitted for investigation. Laboratory evaluation demonstrated a hemogloblin of 8.3 g/dl, platelets count of 41 × 109/l and leucocytes of 5.2 × 109/L of which approximately 30% were circulating promyelocyte with auer rods. Coagulation studies showed an international normalized ratio (INR) of 1.65, activated partial thromboplastin time (aPTT) of 23 seconds and fibrinogen of 77 mg/dL. He promptly received ATRA (45 mg/m2 orally per day fractionated into 2 doses) and supportive care that included cryoprecipitate and platelet transfusions. On the following day a bone marrow aspiration was made showing 84% of promyelocytes, karyotype reveled 15;17 translocation in 4 metaphases (47, X+8[7]/47,idem, t(15;17)[4]/46xy[9]) and fluorescent in situ hybridization test detected PML-RARA fusion.
Along with ATRA the patient received 4 doses of idarubicin 12 mg/m2 on days 3, 5, 7 and 9 days, levofloxacin and fluconazole as antimicrobial prophylaxis.
His treatment course was uneventful until day 9 when he presented with the onset of a fever (axillary temperature of 38.8°C) with no others complaints. There was no weight gain, edema, dyspnea, skin rashes or any other signs or symptoms suggestive of differentiation syndrome.
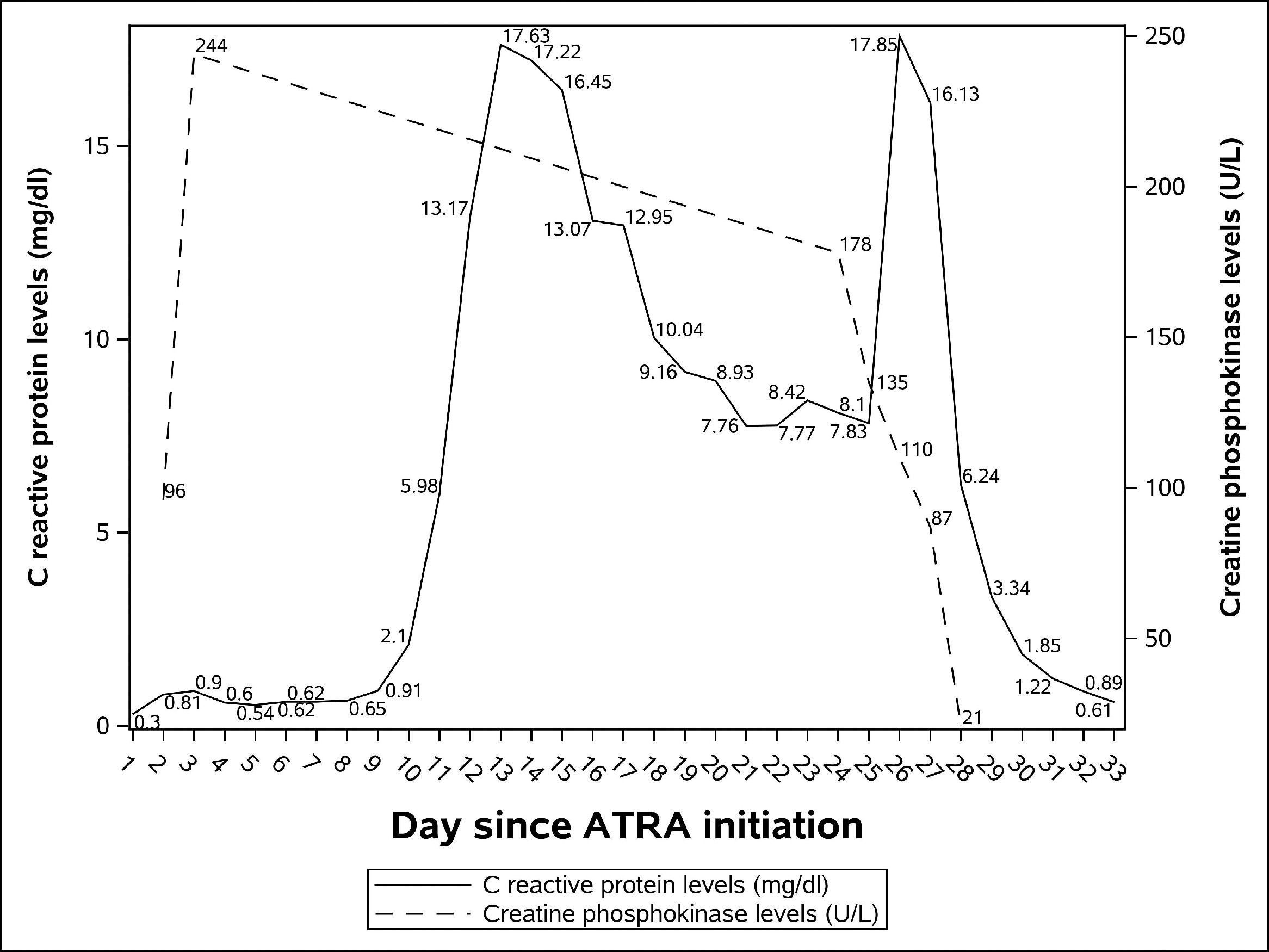
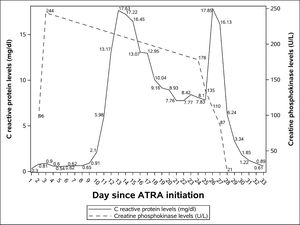
Broad-spectrum antibiotics were started but he remains febrile despite the treatment. Central and peripheral blood cultures were drawn every 48 hours. Urine culture were ordered despite the lack urinary tract symptoms. Contrast-enhanced sinus-chest-abdomen-pelvis computed tomography and galactomannan assays were done. No bacterial or fungal infections were identified. He also underwent transthoracic echocardiography with no evidence of a vegetation indicative of infective endocarditis. Levels of C reactive protein (CRP) rose rapidly since the onset of the fever. Achieving a peak of 17.63 mg/dl (laboratory reference < 0.5 mg/dL) on day 13.
On day 14 he developed a moderate burning sensation while eating. Oral cavity examination showed necrotic ulcers in gingival mucosa. The oral medicine team was contacted and photobiomodulation therapy was performed. By day 20 his pain was gone, the aspect of the oral cavity was better and CRP levels dropped, yet his fever persisted.
Despite the high temperature his clinical condition was excellent until day 24 when he complained of severe pain in his right calf. Physical examination showed redness, swelling, tenderness and pitting edema. No abnormalities were seen in his left leg. Our main hypothesis at the time, deep vein thrombosis, was ruled out with a negative lower limb doppler ultrasound. Creatine phosphokinase levels (CPK) were within normal ranges: 178 U/L (laboratory reference 39-308 U/L). CPK and CRP levels during his admission can be seen in Figure 1.
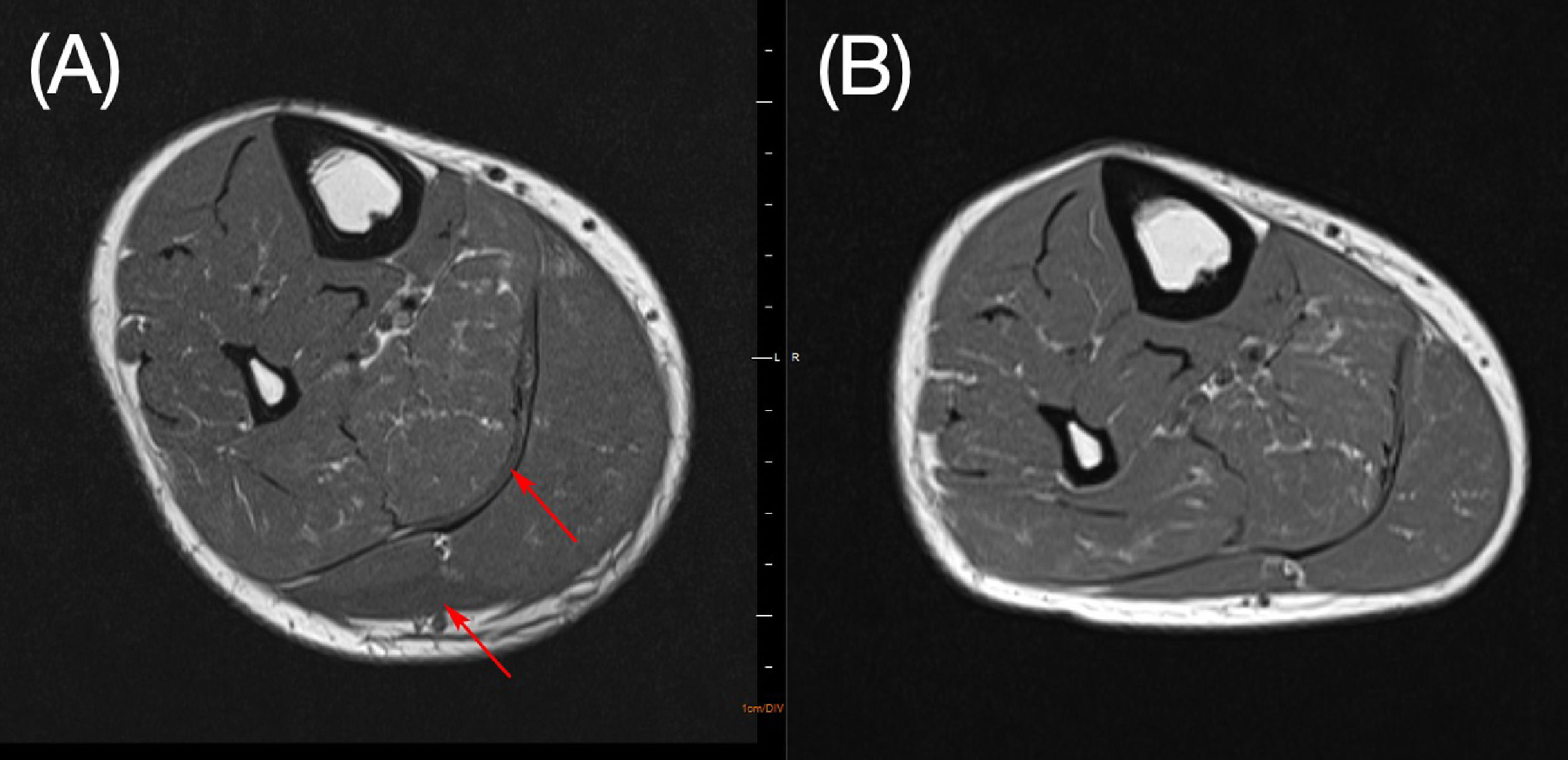
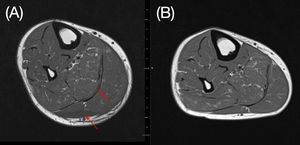
On day 25 his calf pain got worse and CRP levels once more rose, he developed renal dysfunction, hypotension and was transferred to the intensive care unit (ICU). Just before ICU admission a magnetic resonance imaging (MRI) of his right leg was done showing an important volumetric increase in medial and lateral gastrocnemius and mild edema. The findings were suggestive of myositis. We hypothesized that it was an ATRA side-effect.
Consulting the medical literature, we observed that ATRA related myositis was managed in the same way as differentiation syndrome, so on day 26 ATRA was discontinued and therapy with intravenous dexamethasone (10 mg every 12 hours) was started. On the following day his clinical condition improved dramatically; he become afebrile, his renal function got better, his calf redness and swelling were less pronounced and CRP levels dropped. On day 29, 3 days after ATRA withdrawal, the pain had completely gone and all inflammatory signs of his calf had disappeared.
On day 30 ATRA was restarted at usual dose (45 mg/m2 per day). On day 31 he left the ICU and on day 32, as there was no sign of myositis recurrence, there dexamethasone was discontinued without tapering. On day 34 we repeated the MRI with evident improvement in the myositis (Figure 2). A bone marrow aspirate was done showing morphologic and molecular remission. Patient was discharged from hospital on day 35.
ATRA was again used in his consolidation treatment which started 3 weeks after hospital dismissal. At the time of this writing, he is receiving maintenance with 6-mercaptopurine, methotrexate and ATRA. He remains in remission from his leukemia and did not experience myositis recurrence.
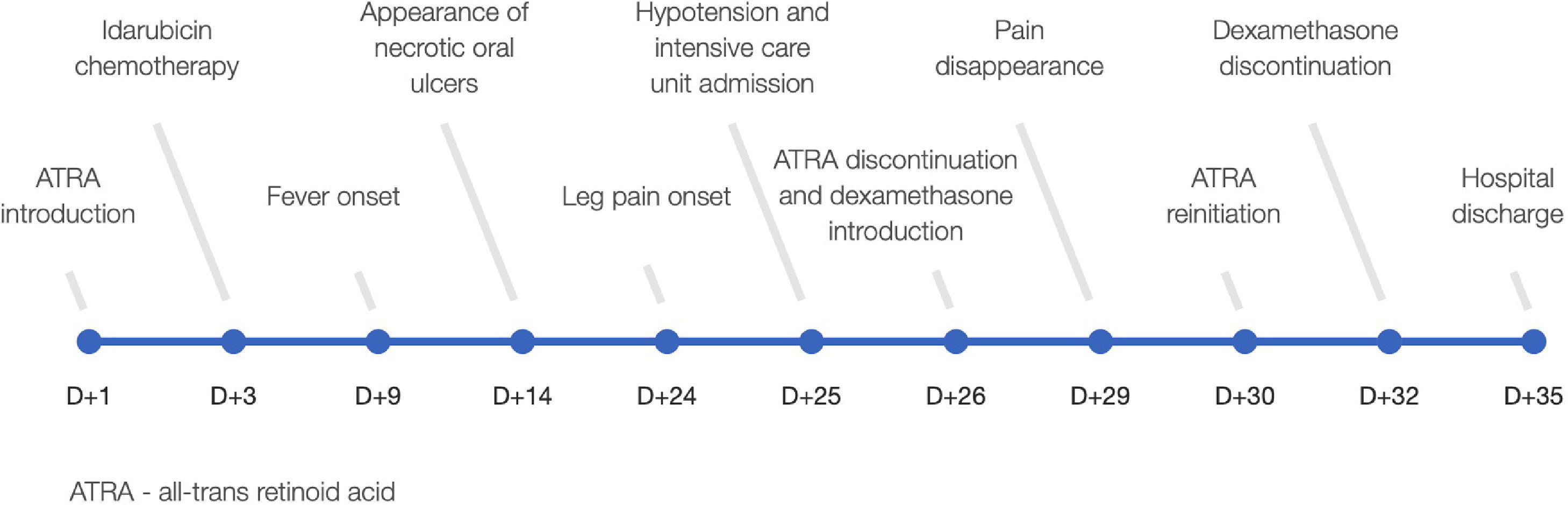
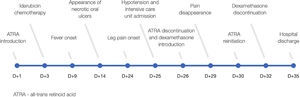
DiscussionATRA has introduced a new era in the management of APL but unfortunately it is not free from serious complications. Here we present a rare and potentially life threatening ATRA side effect. Patient´s main events are summarized in Figure 3.
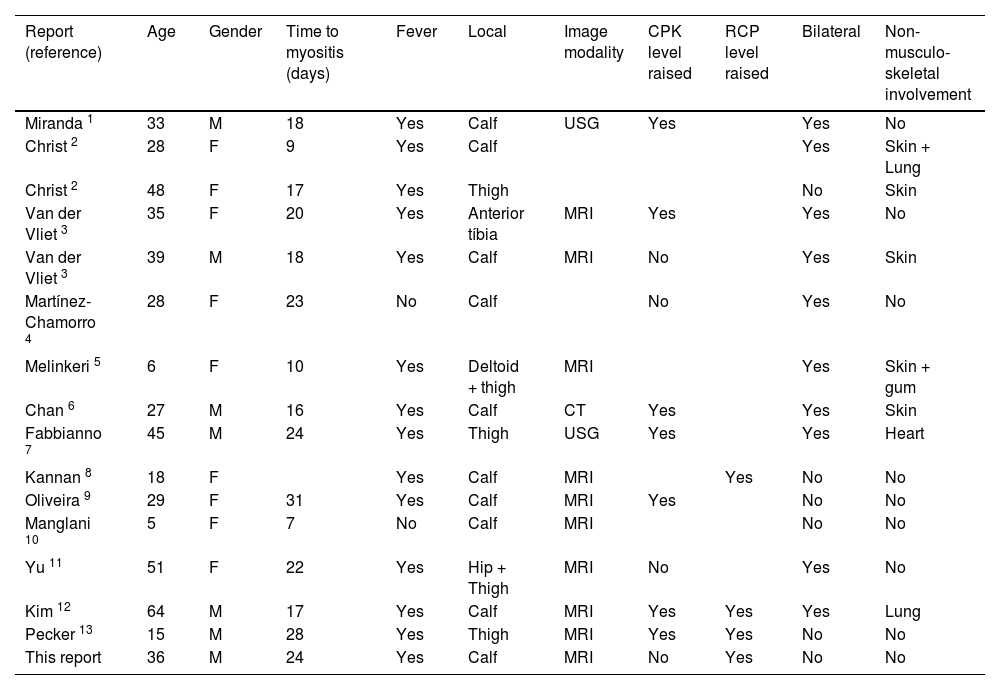
From our literature search, we found 15 reported cases of ATRA-related myositis from 13 articles.1-13 Case reports features are summarized in Table 1. Mean and median age at the onset of ATRA-related myositis (data from the 15 reported cases plus this report) was 31.7 and 31 respectively. Only 7 of the 16 patients were male.
Patients' characteristics of reported cases of ATRA induced myositis.
| Report (reference) | Age | Gender | Time to myositis (days) | Fever | Local | Image modality | CPK level raised | RCP level raised | Bilateral | Non-musculo- skeletal involvement |
|---|---|---|---|---|---|---|---|---|---|---|
| Miranda 1 | 33 | M | 18 | Yes | Calf | USG | Yes | Yes | No | |
| Christ 2 | 28 | F | 9 | Yes | Calf | Yes | Skin + Lung | |||
| Christ 2 | 48 | F | 17 | Yes | Thigh | No | Skin | |||
| Van der Vliet 3 | 35 | F | 20 | Yes | Anterior tíbia | MRI | Yes | Yes | No | |
| Van der Vliet 3 | 39 | M | 18 | Yes | Calf | MRI | No | Yes | Skin | |
| Martínez-Chamorro 4 | 28 | F | 23 | No | Calf | No | Yes | No | ||
| Melinkeri 5 | 6 | F | 10 | Yes | Deltoid + thigh | MRI | Yes | Skin + gum | ||
| Chan 6 | 27 | M | 16 | Yes | Calf | CT | Yes | Yes | Skin | |
| Fabbianno 7 | 45 | M | 24 | Yes | Thigh | USG | Yes | Yes | Heart | |
| Kannan 8 | 18 | F | Yes | Calf | MRI | Yes | No | No | ||
| Oliveira 9 | 29 | F | 31 | Yes | Calf | MRI | Yes | No | No | |
| Manglani 10 | 5 | F | 7 | No | Calf | MRI | No | No | ||
| Yu 11 | 51 | F | 22 | Yes | Hip + Thigh | MRI | No | Yes | No | |
| Kim 12 | 64 | M | 17 | Yes | Calf | MRI | Yes | Yes | Yes | Lung |
| Pecker 13 | 15 | M | 28 | Yes | Thigh | MRI | Yes | Yes | No | No |
| This report | 36 | M | 24 | Yes | Calf | MRI | No | Yes | No | No |
CPK: Creatine phosphokinase; CRP: C reactive protein; M: male; F: female, CT: computed tomography; MRI: Magnetic resonance imaging; USG: ultrasound.
The mean and median time interval between ATRA introduction and myositis was 18.9 and 18 days respectively. Differentiation syndrome onset is more rapid, which has been reported in a range of 7 to 12 days after initiation of ATRA.
All patients had lower limb involvement (16 out of 16 cases) which is often bilateral (10 out of 16 cases). One case had associated deltoid involvement 5 and another one had associated hip involvement.11
Non-musculoskeletal involvement is commonly associated with ATRA-related myositis. 4 cases reported skin involvement,2,3,5,6 1 lung involvement,12 1 heart involvement 7 and 1 skin and lung combined involvement.2 On day 14, our patient evolved with gingival necrosis, it is not clear if it was ATRA-related side effect. Gingival hyperplasia has already been reported with ATRA therapy inclusive associated with myositis.5 However, our patient showed improvement of oral lesions and reduction in CRP values with photobiomodulation and antibiotics even before ATRA withdrawal. Therefore, we do not believe that this manifestation was directly related to ATRA.
ATRA-induced myositis and differential syndrome have many similarities; they both occur 2 to 3 weeks after treatment, are associated with skin involvement and may cause hypotension. Maybe the pathogenesis of both syndromes are similar. ATRA is thought to induce a release of cytokines which can lead to an important inflammatory response. Inflammation is also commonly associated with ATRA-related myositis. All but 2 cases presented fever. In addition to our report, 3 other articles mentioned CRP values all of which were elevated.8,12,13 For that reasons it is possible to hypothesize that myositis is just a spectrum of differentiation syndrome. It is also possible that ATRA related myositis is a "localized" manifestation of differentiation syndrome. Eyes and the heart involvement, for example, have already been described as localized presentations of differentiation syndrome. In our opinion the physiopathological hallmark of myositis and differentiation syndrome are the same: ATRA-induced inflammation.
CPK levels in ATRA-related myositis are not universally elevated. In addition to our case, 3 other reports mention normal CPK levels.3,4,11 Anyway, it is interesting to note that despite our patient's CPK levels were always within normal limits, after treatment, his CPK levels dropped more than 4 times (Figure 1). Therefore, as CPK levels may not help, diagnosis of ATRA-related myosisitis relies upon clinical suspicion and is confirmed with medical imaging. We confirmed the diagnosis with MRI, but ultrasound 7 and computed tomography 6 have already been used with success.
In our opinion this case illustrates very well a rare and important ATRA-related complication which can have devastating effects if it is not rapidly treated. In our case, once myositis was recognized, inflammatory markers and organ damage responded rapidly to a short course of high-dose steroids. This highlights the importance of prompt recognition of this complication, given its potentially life-threatening course early introduction of corticosteroids and ATRA suspension is of utmost importance. Clinicians should be aware that ATRA side effects can go much further than the classical findings of differentiation syndrome.