The prognosis of patients with acute promyelocytic leukemia (APL) has substantially improved with the advent of all-trans retinoic acid therapy (ATRA) and it became, together with anthracycline drugs or arsenic trioxide, a mainstay of the treatment. Despite the effectiveness of ATRA in promoting abnormal promyelocyte differentiation in normal mature granulocytes, up to 27% of the patients that have undergone this therapy may present significant adverse effects such as coagulopathy-induced bleeding or a cytokine-mediated systemic inflammatory response syndrome, named Differentiation Syndrome (DS-APL).1 DS-APL typically occurs in the induction phase, mainly within the first three weeks of treatment. The clinical presentation includes pulmonary infiltrates, fever, bodyweight gain, renal failure, edema, pleural and pericardial effusions and, less frequently, heart failure. Chest pain and acute myocarditis have been rarely described in patients with APL treated with ATRA and may be related to DS-APL. Theoretically, DS-APL may overlap acute anthracyclines cardiotoxicity during the first weeks of treatment for APL. We report the case of a patient with APL submitted to treatment with idarubicin and ATRA therapy during the induction phase that presented acute chest pain due to myocarditis during the course of treatment.
CaseA 57 year-old caucasian man was referred to our institution from a Hospital in Bariloche, Argentina, with the diagnosis of acute promyelocytic leukemia. During his stay at the -first Hospital, the patient started receiving all-trans retinoic acid (ATRA) at a dose of 45mg/m2/daily. Two days later, he was admitted to our institution. The next day after admission, he presented fever with severe neutropenia (190/mm3). Empirical antibiotic therapy (Piperacillin/Tazobactam and Anidulafungin) was started. Three days after he showed fever remission and chemotherapy with idarubicin was started at the dose of 12mg/m2.
On the 17th day of Hospital stay, the patient reported a severe chest pain and was transferred to the Critical Care Unit. The initial physical examination was unremarkable. The first electrocardiogram (ECG) was considered normal, but a subsequent ECG, 5 hours after the beginning of the symptom, revealed T wave inversions in leads DI, AVL, V5 and V6. The laboratory work-up was relevant to an elevated troponin level (3.27 ng/dL, normal range < 0.16ng/dL). A complete view of the laboratory work-up during the hospitalization is shown in Table 1. The first transthoracic echocardiogram showed a preserved left ventricular ejection fraction (LVEF, 71%) with hypokinesis of the basal portion of the septal wall. An initial hypothesis of acute coronary syndrome was made and the patient underwent a coronary angiogram that revealed coronary arteries without significant obstruction. A CT pulmonary angiogram was also performed revealing no filling defect in the pulmonary artery bed. A cardiac magnetic resonance (CMR) was programmed.
Laboratory work-up during the hospitalization.
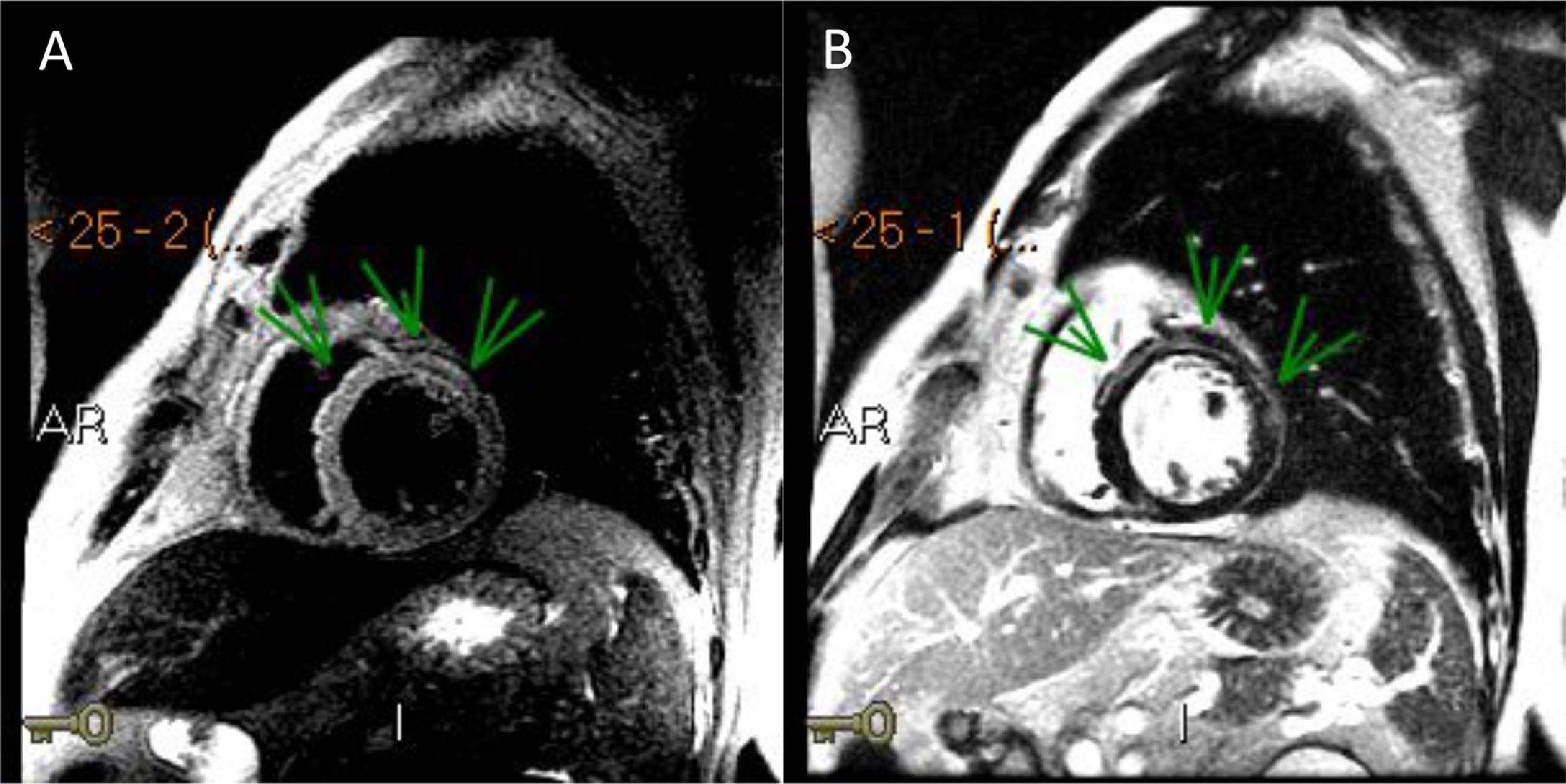
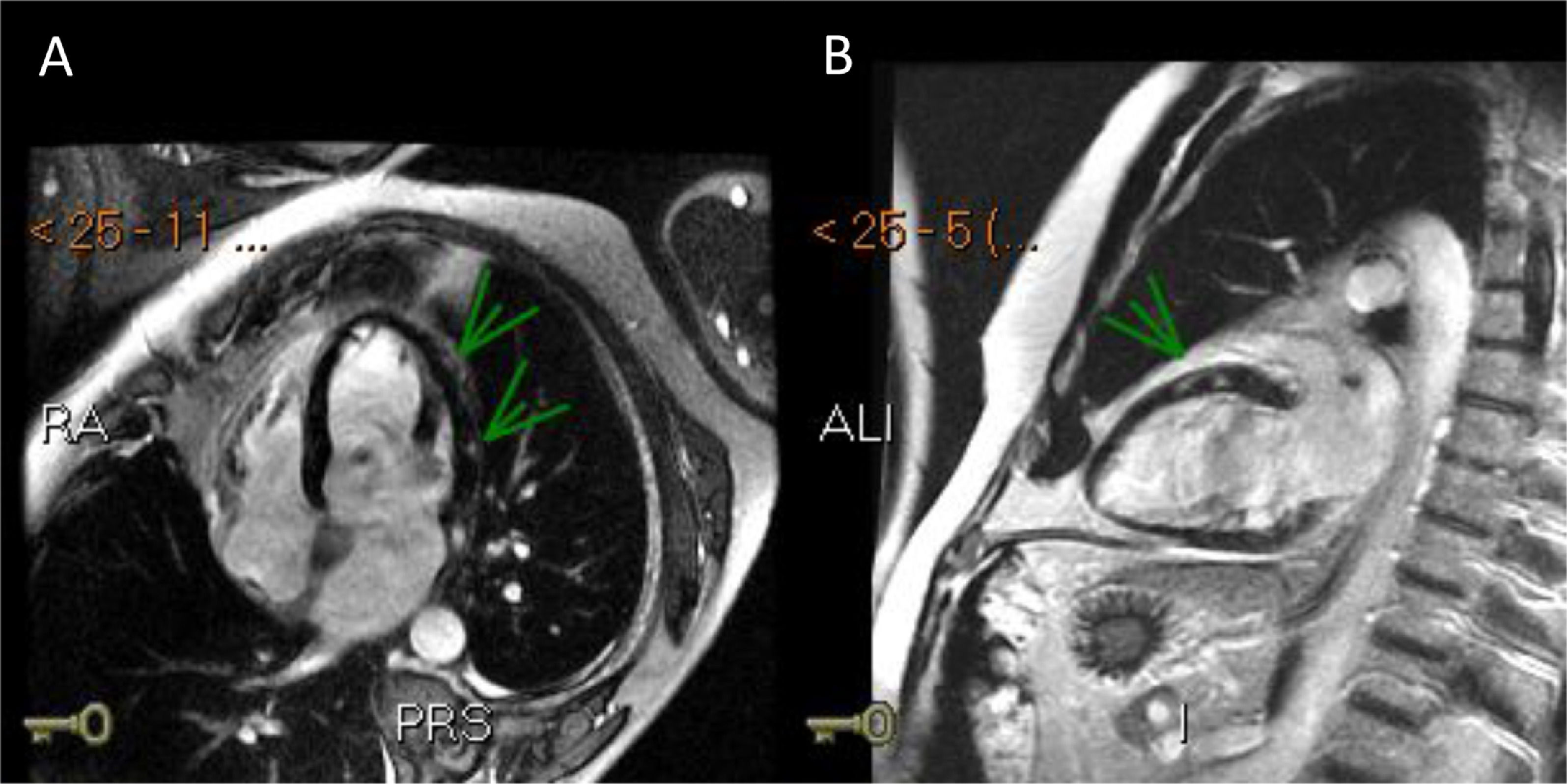
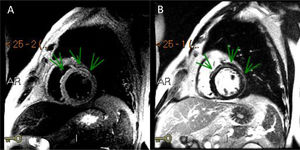
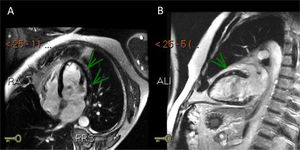
Three days after the first episode of chest pain, the patient had recurrence of the symptom and presented a fever (39ºC). On a physical exam, the patient had slight tachypnea, showing crackles in the lungs, blood pressure 112/72 mmHg, heart-rate 102 bpm and normal capillary filling. No murmur was auscultated in the chest. A pitting edema was noted in both legs. A second echocardiogram showed a LVEF decrease (LVEF 43%) and akinesis of basal portions of septal and inferior walls. Endovenous furosemide and low dose of nitroglycerin were started due to pulmonary congestion. The next day, CMR revealed the left ventricle with increased end-diastolic diameter and reduced contractile function with a LVEF of 42% (NR > 50%). The CMR depicted a marked hypokinesia in basal and mid portions of the anterior, anteroseptal and anterolateral walls and a large subepicardial area of edema and late-enhancement with non-ischemic pattern (Figures 1 and 2). The diagnosis of acute myocarditis was established. ATRA therapy was stopped and the antibiotic therapy scheme was amplified. The patient was already receiving intravenous corticosteroid dexamethasone 10mg daily since the start of the hospitalization . Bacteria and fungi blood cultures, polymerase chain reaction (PCR) for cytomegalovirus, parvovirus B19 and herpesvirus type 6 and serological tests for Epstein-Barr virus and toxoplasma gondii (Igm) were negatives. Coxsackie virus neutralizing antibodies showed small titers for B2, B4 and B5 subtypes, without a rise in titers in another sample that was collected a week ago. A possible association between ATRA therapy and myocarditis was hypothesized.
After the introduction of diuretics (furosemide and spironolactone), beta blocker (metoprolol succinate) and ATRA therapy stoppage, the patient's clinical status improved with resolution of pulmonary congestion, tachycardia and fever. A new echocardiogram performed two weeks later showed a preserved LVEF (55%) and normal wall motion. During the consolidation phase, chemotherapy with trioxide arsenic was started and ATRA therapy was retaken. During the 12 months follow-up, the patient remained asymptomatic without evidence of heart failure or fever recurrence.
DiscussionDue to the manifestation of chest pain in a 52 years-old man with hematologic cancer, increased levels of troponin and dynamic electrocardiographic changes, we initially opted to rule-out acute coronary syndrome and pulmonary embolism. During evolution, the onset of fever and signs of heart failure raised suspicion of acute myocarditis, which was confirmed by cardiac resonance.
Few reports of acute myocarditis have been described in patients with APL under treatment with ATRA.2–6 In most cases, the diagnosis of myocarditis came together with suggestive signals of DS-APL, which is related to ATRA-induced systemic inflammatory response syndrome, endothelium damage with capillary leak syndrome, occlusion of microcirculation, and tissue infiltration.7 The onset of symptoms related to myocarditis in these reports usually started during the first three weeks of treatment with ATRA, which is also commonly the onset time of the DS-APL. Interestingly, van Rijssel et al reported the autopsy findings of a patient with APL that suffered a sudden death related to acute myocarditis during the ATRA therapy. The autopsy revealed fibrinous pericarditis and extensive myocarditis with inflammatory infiltrates of cells of the myeloid series, which were proven to belong to malignant APL clones with the help of hybridization and immunohistochemistry techniques.8 In our patient, some heart failure symptoms and signals, such as pulmonary infiltrates, body weight gain, respiratory distress and edema, overlapped with DS-APL patterns.
Acute/early onset cardiotoxicity due to anthracyclines, unlike chronic/late onset cardiotoxicity, is a rare manifestation and is generally characterized as serious illness with severe myocardial dysfunction, refractory heart failure and, in so many cases, leading to death.9 Early onset cardiotoxicity may occur anytime in the early stages of idarubicin therapy, is dose-dependent, and beyond congestive heart failure, supraventricular and ventricular arrhythmias are also frequently observed. In a series of 165 patients with acute leukemia and without previous heart disease treated with anthracyclines between 1990 and 1999 in a single center, seven patients developed early cardiotoxicity and severe heart failure that resulted in 6 deaths associated to acute cardiotoxicity.9 Our patient received a comparative low total dose of idarubicin and had a prompt recovery from heart failure. However, a role of idarubicin therapy on the myocardial damage onset in this case is possible and plausible.
Acute myocarditis has been related with several causes such as viral and bacterial infections, autoimmune diseases, drug toxicities and others. In immunocompromised patients, some infectious agents may cause myocarditis such as cytomegalovirus, toxoplasma gondii and reactivation of Chagas’ disease. Despite the lack of endomyocardial biopsy in our case, serologies and PCRs for the most common infectious agents linked to myocarditis weren't suggestive of an infectious cause.
In conclusion, we reported a case of acute myocarditis in a patient with acute promyelocytic leukemia treated with ATRA and idarubicin. Though extensive investigation, no other agents than ATRA and anthracycline were identified as causative factors. In this clinical scenario, ATRA therapy was considered as the probable causative factor for acute myocarditis. However, acute cardiotoxicity due to anthracycline therapy may have also had a role in the myocarditis onset in this case. Of note, our report is one of the first documentations with cardiac magnetic resonance imaging of myocardial injury due to ATRA and anthracycline-induced myocarditis. As other reports in literature, the clinical presentation in our case suggested an intersection between the clinical aspects of the Differentiation Syndrome and cardiac involvement with heart failure during ATRA therapy in patients with APL.
EthicsThe informed consent was obtained and the research was approved by the Hospital Institutional Review Board. The patient's privacy rights have been guaranteed.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.