Chronic Myeloid Leukemia (CML) is a myeloproliferative disease that affects mainly adults between 50 and 55 years. In Brazil, information from the Sistema Único de Saúde (SUS) Outpatient Information System indicates that 12,531 patients had the Autorização de Procedimento Ambulatorial (APAC) approved for the CML treatment in 2017. Disease monitoring through molecular response evaluation is critical to the care of CML patients. The quantitative PCR test (real-time polymerase chain reaction) provides adequate evaluation parameters that allow the health professional to intervene at the right moments in order to reduce the chance of progression of the disease, providing the best outcome to the patient, including the possibility of treatment discontinuation for eligible patients. Although the test is included in the Clinical Protocol and Therapeutic Guidelines (PCDT) of CML, it is not possible to monitor the molecular response within SUS since there is no reimbursement for this test.
ObjectiveObtain expert recommendations on the importance, financing, and reimbursement of molecular monitoring in SUS.
MethodsSix CML experts with different perspectives participated in the panel. The discussion was based in the main publications about the quantitative PCR test in CML monitoring.
ResultsExperts’ recommendations:
- 1)
Molecular monitoring should be part of the integral treatment of patients with CML to reduce the chances of disease progression and costs to the health system;
- 2)
The government should put into practice what is provided in the PCDT of Chronic Myeloid Leukemia in Brazil: performing the monitoring of the molecular response via quantitative PCR;
- 3)
The government should create a code with adequate nomenclature and reimbursement value in SIGTAP, so that the test is carried out and covered by the public health network, as it is contained in the PCDT of the disease and the existing APAC does not cover the operational costs for its performance;
- 4)
Patients with chronic phase CML should perform a quantitative PCR every 3 months and, after reaching the MMR, should perform the examination every 6 months, as recommended by international guidelines;
- 5)
Patients should be monitored in reference laboratories that are standardized according to the international scale;
- 6)
The laboratories that are within the reference public centers could absorb all the test demand in Brazil, and other centers could be qualified through an ABHH accreditation;
- 7)
Adequate molecular monitoring may allow some patients to stop taking drugs and selffinancing the molecular test for all SUS patients
A solution for the molecular test (BCR-ABL1) funding is urgent to ensure the monitoring of CML patients in SUS. The savings that might be generated with patients that stop taking the medication when adequately monitored may finance the test.
Chronic myeloid leukemia (CML) is a myeloproliferative disease characterized by the excessive accumulation of mature cells of the myeloid lineage.1 CML is distinguished from other types of leukemia by the presence of a genetic abnormality in the white blood cells called Philadelphia chromosome (Ph+). In patients with the disease, there is a translocation (fusion of one part of a chromosome into another chromosome) between two chromosomes, number 9 and 22, resulting in the fusion of the genes BCR and ABL (BCR-ABL), thus characterizing chronic myeloid leukemia.2
At first, the disease develops slowly and affects mostly adults,3 especially between 50 and 55 years of age. Worldwide, the incidence is 1–1.5 patients per 100,000 inhabitants per year. In Brazil, in 2012, information from the Brazilian public healthcare system (SUS) Outpatient Information System (SIS) pointed to an annual prevalence of around 10,125 cases of CML. Brazilian data also indicate that the age range of the disease is 10 years younger than the international data, with median age at diagnosis between 40 and 46 years.1
The main symptoms are: anemia, tiredness and malaise, enlarged spleen (splenomegaly), excessive sweating, weight loss and bruising.2 Most patients are asymptomatic (30%–50%), and the disease is discovered through routine exams.4
In case of CML suspicion, blood count is the first exam to be requested for diagnosis – in positive case, the number of leukocytes will be altered. Diagnostic confirmation is made by examining the bone marrow (myelogram) considering cytological, cytogenetic and immunophenotypic analyses.3 Additionally, the molecular polymerase chain reaction (PCR) test is used qualitative for diagnosis of BCR-ABL detection and also used quantitative to measure BCR-ABL transcripts to follow up treatments2 and collected in peripheral blood.
The assessment of complete hematologic response is based on complete blood count, which usually occurs within the first three months of treatment. Complete response is considered when blood cell values return to normal, there are no more immature blood cells and the spleen size normalizes.5,6
Complete cytogenetic response is detected in cases no cells are observed with the Ph chromosome in the bone marrow and this response must be obtained within 6 months of treatment, according to the European LeukemiaNet (ELN).6 In order to obtain these results, a bone marrow test is necessary, and the cytogenetic or fluorescence (FISH) test can be performed.
The hybrid gene BCR-ABL is responsible for encoding a protein with high tyrosine kinase activity that leads to the molecular process that determines the transformation of the normal hematopoietic progenitor cell into malignant.2 The advent of tyrosine kinase inhibitors (TKIs) has revolutionized the treatment of CML – these molecules act by inhibiting the BCR-ABL1 protein from playing its role in oncogenesis, thus preventing disease progression and resulting in a lower chance of progression to accelerated phases and higher survival of patients. Currently there are inhibitors approved for both first-line (imatinib) and second-line (nilotinib and dasatinib) treatment for patients resistant or intolerant to initial therapy. In this context, the monitoring of BCR-ABL levels in order to identify resistance to treatment and to prevent disease progression and death is very important.7
For CML treatment, European LeukemiaNet (ELN)8 recommends the evaluation of hematological, cytogenetic and molecular response criteria, which indicates the response to treatment.
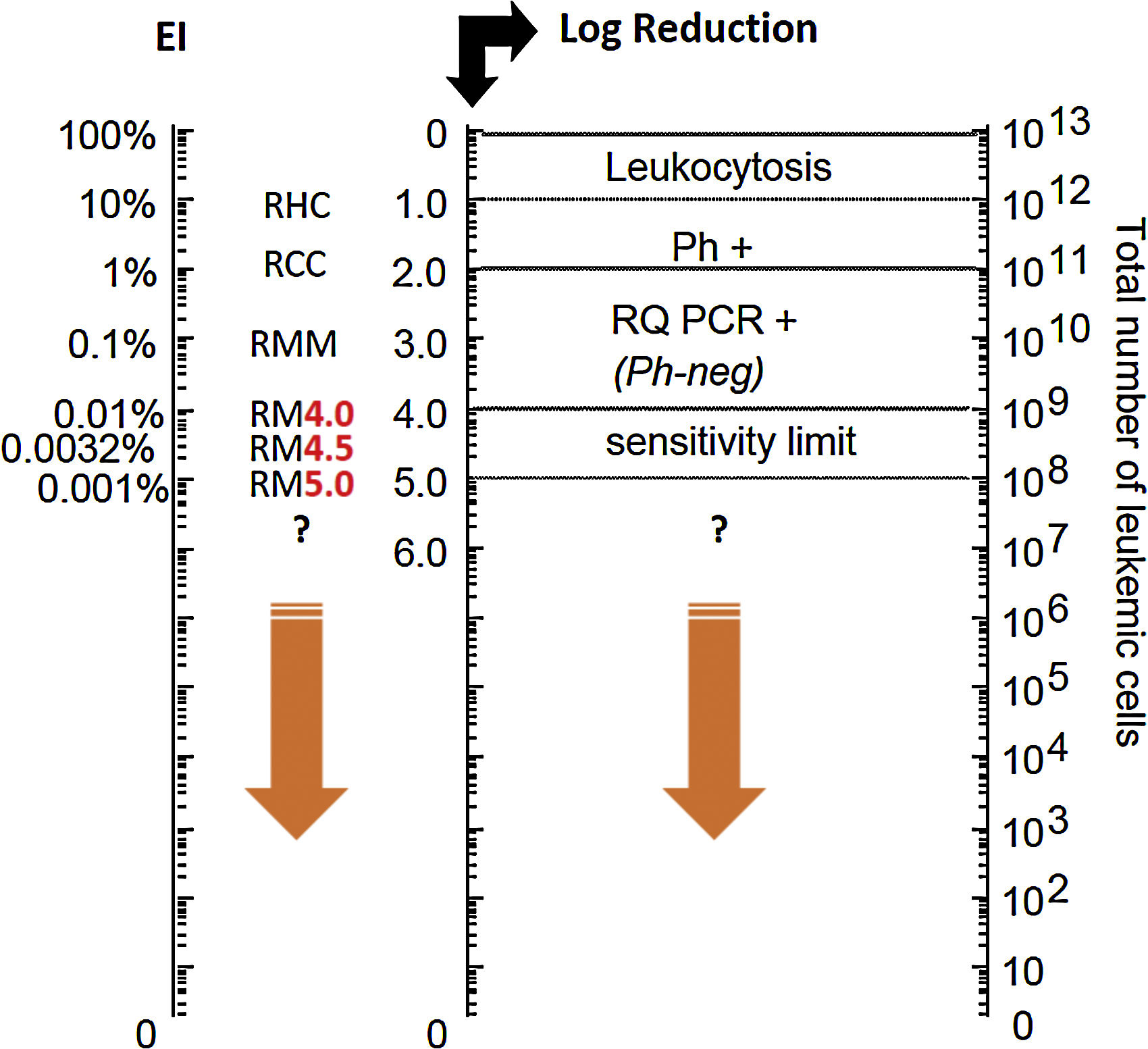
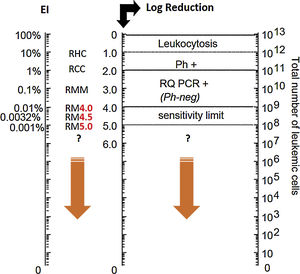
Considering its central role in the CML pathogenesis, quantification of the number of BCR-ABL gene transcripts through quantitative molecular monitoring by PCR analysis is the most sensitive marker of the efficacy of treatment with TKI (Fig. 1). The main objective of treatment according to ELN is the major molecular response (MMR) – a reduction below 0.1% of the number of BCR-ABL gene transcripts after 12 months of first-line therapy, with this evaluation being possible only by using the PCR test. Molecular analysis of BCR-ABL levels by PCR is recommended every 3 months until MMR is reached. After this period, molecular monitoring can be performed every 6 months.6 The sensitivity of the molecular test is at least two logs higher than the conventional cytogenetics (Fig. 1).
Leukemic load (BCR-ABL ratio)/baseline log reduction ratio.
Adapted from: Luu at al. (2013).9 Ph+: Philadelphia chromosome; RQ PCR+: real time PCR; RHC: complete hematologic response; RCC: complete cytogenetic response; RM: molecular response; RMM: major molecular response.
Deep molecular response, i.e., a reduction of 0.01% (RM 4 logs) of BCR-ABL levels, has been gaining importance in the treatment of CML, being an optimal response marker at 18 months of treatment according to the last update of the European Society for Medical Oncology (ESMO) guidelines of 201710 for patients whose goal is to discontinue treatment. The extent of the response is important, as patients achieving a deep molecular response do not progress, and is the main criterion used in TKIs discontinuation studies.10
In these discontinuation studies,10 patients sustaining the deep response (RM4 or RM 4.5) after a few years of treatment with TKI discontinued treatment and were strictly monitored by PCR examination. The success rates of these studies demonstrate that 40–50% of patients meeting eligibility criteria for discontinuation can sustain treatment-free remission. The discontinuation of TKIs brings many benefits for patients, mainly due to the reduction of adverse events, higher quality of life and feeling cured from the disease. In addition to the benefits to the patient, treatment discontinuation promotes an economy for the health system. Currently the discontinuation of TKIs is already recommended in clinical practice by the NCCN and ESMO guidelines.
The Ministry of Health provides first line treatment through imatinib mesylate and for patients intolerant or resistant to this TKI (about 30–40%), there is the possibility of switching to second line treatment through more potent second-generation inhibitors, nilotinib and dasatinib. According to the current Clinical Protocol and Therapeutic Guidelines (PCDT) for Chronic Myeloid Leukemia,1 the PCR test will be done for diagnosis and monitoring, after confirmation of the complete cytogenetic response. However, PCR test is not included in the SIGTAP table,11 either in code form or in descriptive form related to Chronic Myeloid Leukemia preventing the procedure reimbursement and use by the main centers. Given this fact, it is impossible to evaluate molecular responses that are crucial for the identification of patients who do not respond to first-line treatment and who need to change treatment in order to avoid disease progression.
Therefore, it is understood that first-line and second-line treatments are made available by the public health system, however; the access to the most sensitive examination recommended by international guidelines for response evaluation is not performed as recommended by PCDT.1
MethodsPanel of experts and consensus methodologyThe first step involved a team of health consultants who conducted a literature review to identify key publications on the role of quantitative PCR (BCR-ABL) in the monitoring of Chronic Myeloid Leukemia (CML).
Then, in order to increase the technical-scientific arsenal, a list with names of several specialists (physicians, managers and representatives of patient associations), who could compose a consensus panel about the impact of said test in clinical practice, was selected.
Finally, six individuals participated in the panel, namely: three hematology physicians from the main public institutions that treat patients with CML in the country (INCA and HEMORIO), a coordinator physician from the laboratory who standardized the PCR test for BCR-ABL in Brazil, located at the School of Medicine of USP-SP, a hematologist who belongs to Hospital Alemão Oswaldo Cruz (HAOC), a representative of the Brazilian society of pharmacists in Oncology (Sociedade Brasileira de Farmacêuticos em Oncologia – SOBRAFO) and a hospital unit manager (Hospital de Clínicas de Porto Alegre/UFRGS) who has specialized care for these patients, and a representative of the largest association of patients with leukemia in Brazil (ABRALE). The diversity of participants was an essential factor in the elaboration of this consensus, since all areas that comprise the global treatment of the disease (diagnosis, treatment, monitoring, resources availability and social representativeness) were contemplated in this process.
Questions to be answeredFrom the scientific findings, five central questions were elaborated to understand the clinical and economic impact of BCR-ABL test by quantitative PCR in CML monitoring.
The panel was held on April 3rd, 2019, in São Paulo. Each question was extensively debated through discussion rounds, and a draft was established for the answers to each question. Subsequently, the panel weighed all the information gathered in the discussion rounds and reached what was considered a complete consensus statement addressing all five pre-determined questions.
The consensual answers to the following five questions are described in the sections below, as well as the recommendations on the implementation of the test in Brazil and its importance in the monitoring of CML.
What is the importance of molecular monitoring with BCR-ABL in the treatment of CML patients?There are three important tests that must be performed by the healthcare professional to assure that the CML patient is responsive to treatment with TKIs (imatinib, nilotinib or dasatinib):
- •
Complete blood count
Objective: complete hematological response (CHR) at three months – which means that the number of white cells, red cells and platelets are normalized.12
- •
Cytogenetic examination
Objective: complete cytogenetic response (CCR) at 6 months – which means the absence of cells presenting the Philadelphia chromosome in 20 metaphases analyzed by the cytogenetic test.6
- •
Quantitative PCR examination
Objective: major molecular response (MMR) at 12 months – which means that the number of BCR-ABL gene transcripts decreased to less than 0.1%, according to the international scale.6
Molecular analysis is indicated to monitor molecular responses that define the percentage of BCR-ABL+ neoplastic cells present in CML patients during different treatment phases.
The monitoring at every three months that is indicated by the PCDT1 and supported by international guidelines6,13,14 can provide important prognostic data for decision making in CML patients, performing the appropriate therapeutic interventions to avoid disease progression. The progression of CML can be fatal, after disease progression, the median survival is only 10 months, and the main objective of treatment is to keep patients in the chronic phase of the disease.
Over 70% of patients may achieve complete cytogenetic remission, and 20%–40% will show suppression of BCR-ABL transcript levels, leading to the achievement of the major molecular response (<0.1% in the international scale) at the end of the first year of treatment.15
In most Brazilian institutions, it is not possible to carry out molecular monitoring (as recommended by international guidelines6,14) despite its description in the Clinical Protocol and Therapeutic Guidelines (PCDT1) of the disease, since the test does not have a code within the Management System of Procedures, Medications and OPM Table of SUS (SIGTAP).11 Currently, this molecular monitoring is carried out in private institutions or university hospitals of great complexity by initiative of the institution itself or through vouchers offered by the private initiative. Thus, the absence of molecular examination access for all patients hurts the principle of equity and integrality of SUS.
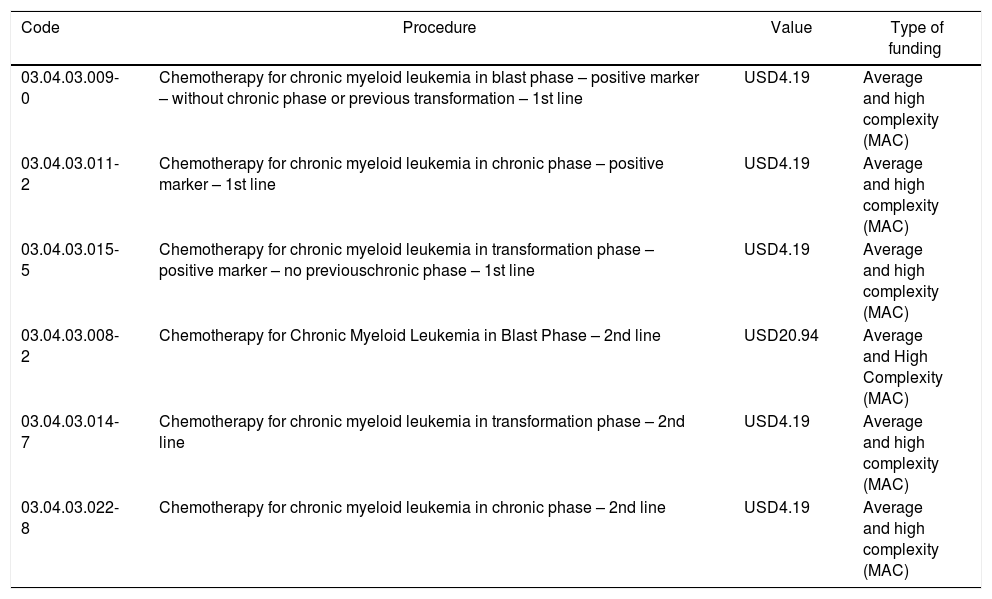
The reimbursement structure of oncology therapy in SUS also influences the process of covering the test in the country. In the 1990s, the Ministry of Health gathered its internal technical bodies for a complete review of all regulations and tables of oncological procedures.16 Based on this effort, and under the basic principles of integral and integrated assistance to the Brazilian population, updating procedures and generating data for result evaluation, the Ministry of Health published several regulations to standardize procedure authorizations (APACs) in Oncology. In the specific case of Chronic Myeloid Leukemia, the current regulation presents a reimbursement value of USD 4.19 (Table 1) – which precludes the funding of molecular monitoring.
APAC procedures and corresponding amounts changed by Ordinance No. 90 of March 15th, 2011 and by Ordinance No. 103 of January 30th, 2015.
| Code | Procedure | Value | Type of funding |
|---|---|---|---|
| 03.04.03.009-0 | Chemotherapy for chronic myeloid leukemia in blast phase – positive marker – without chronic phase or previous transformation – 1st line | USD4.19 | Average and high complexity (MAC) |
| 03.04.03.011-2 | Chemotherapy for chronic myeloid leukemia in chronic phase – positive marker – 1st line | USD4.19 | Average and high complexity (MAC) |
| 03.04.03.015-5 | Chemotherapy for chronic myeloid leukemia in transformation phase – positive marker – no previouschronic phase – 1st line | USD4.19 | Average and high complexity (MAC) |
| 03.04.03.008-2 | Chemotherapy for Chronic Myeloid Leukemia in Blast Phase – 2nd line | USD20.94 | Average and High Complexity (MAC) |
| 03.04.03.014-7 | Chemotherapy for chronic myeloid leukemia in transformation phase – 2nd line | USD4.19 | Average and high complexity (MAC) |
| 03.04.03.022-8 | Chemotherapy for chronic myeloid leukemia in chronic phase – 2nd line | USD4.19 | Average and high complexity (MAC) |
1USD=4.06BRL (17.12.2019).
The BCR-ABL test (quantitative PCR), as already mentioned, does not have an exclusive code in SIGTAP (Management System of the Procedures, Medications and OPM Table of SUS), which promotes difficulties in its conduction quantification and valuation. In addition, specialized and properly qualified laboratories should receive the patients’ samples and perform the test quarterly – in this way, it would be possible to quantify the number of tests and patients attended, contributing to the proper follow-up of the patient during the treatment.
Experts agree that the test is an intrinsic part of the treatment for Chronic Myeloid Leukemia because it would allow the proper follow up of the patients and quantify their quarterly responses through a high sensitivity test. In the absence of molecular monitoring, it is not possible to have a truly effective treatment, exposing these patients to the risk of disease progression and death.
Therefore, the group reached the consensus that it is necessary to monitor adequately the patient to ensure the best outcome in each case. This outcome is only achieved if the patient is monitored, enabling appropriate intervention at a timely manner.
Molecular monitoring is already reported in PCDT (Clinical Protocol and Therapeutic Guideline) of CML. In this scenario, how is the monitoring of patients with CML who are being treated in SUS?Chronic Myeloid Leukemia (CML) already has a “Clinical Protocol and Therapeutic Guideline” (PCDT)1 for guidance and patient care under SUS, as previously presented. According to this PCDT, updated in 2015, the understanding of CML pathophysiology and the improvement of diagnostic means introduced new concepts in patient monitoring – clinical response, hematological response, cytogenetic response and molecular response.
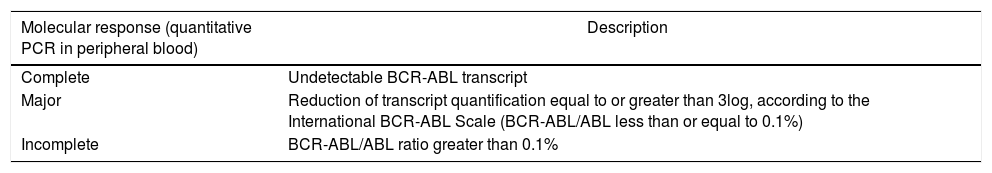
The molecular response of PCDT is defined as described in Table 2.
Molecular response defined in CML PCDT.
| Molecular response (quantitative PCR in peripheral blood) | Description |
|---|---|
| Complete | Undetectable BCR-ABL transcript |
| Major | Reduction of transcript quantification equal to or greater than 3log, according to the International BCR-ABL Scale (BCR-ABL/ABL less than or equal to 0.1%) |
| Incomplete | BCR-ABL/ABL ratio greater than 0.1% |
Also according to this PCDT,1 some tests should be performed to monitor the patient. Among them is the molecular examination, described and defined periodically as follows:
Molecular examination (quantification of BCR-ABL transcripts by RT-PCR)- -
Every three months after cytogenetic response obtained and confirmed.
- -
After obtaining and confirming major molecular response, it can be performed every six months.
- -
Perform on peripheral blood samples.
The experts believe that the optimal number of tests that should be performed for CML patients includes:
- •
1 qualitative PCR at the time of diagnosis;
- •
1 quantitative PCR every 3 months (monitoring);
- •
After achieving major molecular response (MMR), perform quantitative PCR every 6 months;
It is noted that the schemes in question are similar – however, according to the text of the current PCDT, it would only be possible to perform quantitative PCR after complete and confirmed cytogenetic examination. However, it is understood that the fulfillment of the current PCDT in relation to execution of diagnostic tests in full is crucial for CML patients, as the noncompliance of the monitoring provided in this protocol (as the test is not offered by SUS), has great impact on the treatment of these patients.
Thus, it would be important to formalize the inclusion and a proper nomenclature and code for the BCR-ABL test (with the same description of PCDT – quantification of BCR-ABL1 transcripts by RT-PCR) in SIGTAP, so that the public health system could officially fund this test in the care network.
Does SUS have technical and operational capacity to execute and implement BCR-ABL molecular monitoring, given the volume of patients?The experts consulted found that 95% of CML patients in SUS are concentrated in large treatment centers (CACONs/UNACONs).
However, experts acknowledge that the BCR-ABL test is performed in highly complex hospitals today (mainly university and/or research hospitals), such as: Hospital das Clínicas – USP, Hospital das Clínicas de Ribeirão Preto – USP Ribeirão Preto, Hospital das Clínicas da Universidade Estadual de Campinas – UNICAMP, Hospital das Clínicas da Universidade Estadual Júlio de Mesquita – UNESP, Hospital de Clínicas de Porto Alegre/UFRGS and Hospital de Clínicas de Curitiba da Universidade Federal do Paraná. From the expert perspective, it is understood that SUS would have the technical and operational capacity to absorb the total national demand.
The experts suggested an implementation of a technical staff (supported by the society) that could be responsible for accreditation, management and quality of new laboratories, in order to increase the technical and operational capacity of the current public health system.
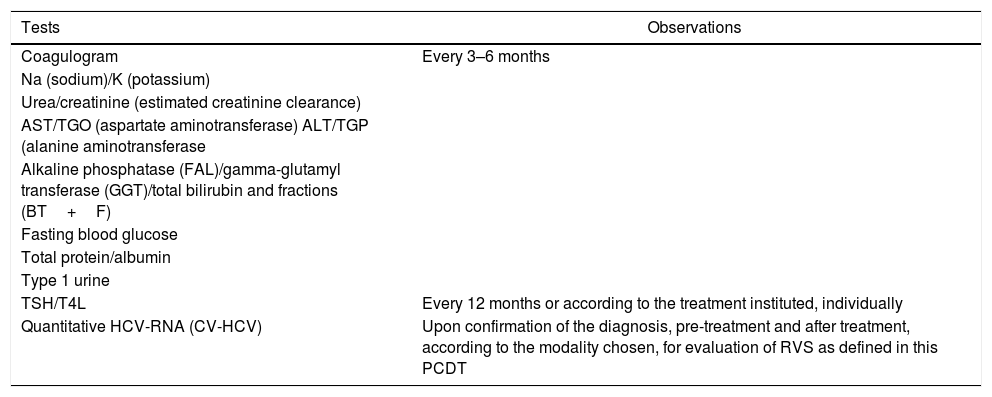
Other diseases, such as hepatitis C, have a protocol that addresses the specific monitoring of the disease through predetermined clinical exams. PCDT for Hepatitis C and Co-infections17 defines that the initial and routine laboratory approach of patients with chronic hepatitis C has several purposes, such as defining the moment of treatment initiation, establishing the recommended therapeutic regimen, evaluating the quality of the response obtained with the therapeutic strategy and assisting in cancer tracing. In order to facilitate clinical monitoring of patients with chronic hepatitis C and to assist in the best use of technical and financial resources, PCDT indicates a list of complementary tests (Table 3).
Some complementary tests to monitor hepatitis C.
| Tests | Observations |
|---|---|
| Coagulogram | Every 3–6 months |
| Na (sodium)/K (potassium) | |
| Urea/creatinine (estimated creatinine clearance) | |
| AST/TGO (aspartate aminotransferase) ALT/TGP (alanine aminotransferase | |
| Alkaline phosphatase (FAL)/gamma-glutamyl transferase (GGT)/total bilirubin and fractions (BT+F) | |
| Fasting blood glucose | |
| Total protein/albumin | |
| Type 1 urine | |
| TSH/T4L | Every 12 months or according to the treatment instituted, individually |
| Quantitative HCV-RNA (CV-HCV) | Upon confirmation of the diagnosis, pre-treatment and after treatment, according to the modality chosen, for evaluation of RVS as defined in this PCDT |
TSH: thyroid-stimulating hormone; T4L: circulating free thyroxine; HCV: hepatitis C virus; RNA: ribonucleic acid.
The clinical monitoring in the public health system is already observed as a reality – this action allows the patient to be properly followed during the course of the disease and helps the best use of technical and financial resources of the system. In addition, it is known that early intervention upon the patient's clinical worsening allows quick actions that improve the patient's quality of life.
What are the clinical and economic benefits of incorporating the BCR-ABL molecular test into SUS?Clinical benefitsQuantitative PCR (BCR-ABL) monitoring is an important tool for monitoring the patient with CML during the recommended treatment.
The quarterly molecular monitoring until MMR and then at every 6 months,6 as recommended by the ELN, allows the patient with CML to be adequately assisted throughout the treatment period. This provides adequate evaluation parameters for the healthcare professional, who can intervene in the right moments to provide the best outcome to the patient.
Early intervention against the altered parameter would enable a reduction in disease progression to accelerated phases and increase survival, as reported by specialists. In a retrospective review of 402 patients with CML treated with first line imatinib, patients performing molecular monitoring 3–4 times a year had a disease progression rate of 2% versus 15% for patients who performed no tests per year, demonstrating the importance of monitoring the outcome of CML. With the current treatment, if well monitored, patients with CML may have a life expectancy close to the normal population.
The experts also pointed out that molecular monitoring promotes treatment benefits because the patient should go to the reference center for the test every three months at the beginning of therapy. Therefore, it would be possible to evaluate adherence to treatment, besides obtaining administrative information of public interest, such as: the frequency with which patients return to the reference center, how many tests are performed, monthly cost, among others. In a retrospective study based on the statements of patients with CML in therapy with first-line TKI (N=1205), which assessed adherence to treatment for 12 months and demonstrated that patients with 3–4 molecular tests per year have an 85% treatment adherence rate, against 77% of those who did zero monitoring per year.
The work capacity of the patients could also be rescued, as recent studies18,19 showed that patients with sustained deep molecular response (performed via quarterly monitoring) during the one-year consolidation phase of tyrosine kinase inhibitor were eligible to discontinue treatment and enter remission phase.
Economic benefitsExperts have pointed out that disease progression is costly for the public healthcare system, as it leads to recurrent hospitalizations, transplants, new treatments with other lines of medication, reduced productivity, work leave, and others.
The experts achieved consensus on the importance of monitoring being linked to the possibility of discontinuing treatment with TKIs, according to results of the studies mentioned above, which currently is a clinical practice recommended by the guidelines of ESMO and NCCN, and it would benefit not only patients but also reduce costs for the health system. The discontinuation study STIM (STopIMatinib), which has the longest follow-up to date, included 100 patients who discontinued the use of imatinib after sustaining a deep molecular response for at least 2 years, 38% of whom remained more than 8 years without use of medication, maintaining treatment-free remission. However, without access to PCR examination, it is not possible to benefit patients with the treatment discontinuation possibility.
What are the main strategies that could finance molecular testing in SUS?The experts agreed by consensus that the existence of a code in SIGTAP, with adequate nomenclature, is the best strategy for financing the molecular test in SUS, as the PCDT contemplates the test in the detailed monitoring of the disease and the current APAC value precluded the proper conduction of the test in specialized laboratories and/or authorized reference centers.
From the economic point of view, the incorporation of the test could reduce costs considering the currently recommended treatment for CML, since specialists work with the premise that if molecular monitoring is done properly, the patient follow up in order to prevent disease progression and death is possible; besides electing patients with sustained deep response to treatment discontinuation.18,19 This is an argument supported by the experts regarding the negotiation of incorporation of the test in SUS, as other countries have already taken this step, according to data of said studies.
In 2018, during the congress of the American Society of Hematology (ASH), a study published by leading physicians treating CML in Brazil demonstrated that discontinuation of 1 of the 3 TKIs available in SUS could generate savings for the Brazilian health system possible of paying for PCR tests for all patients with CML in the country. Finally, it is important to educate patients and physicians on the possibility of discontinuing of treatment through explanation of all treatment steps, carrying out the adequate quarterly follow-up and planning the discontinuation at the optimum point recommended by the presented studies.18,19
ConclusionThe study concluded that the incorporation of the quantitative PCR test (BCR-ABL) is fundamental for adequate treatment of chronic myeloid leukemia in the country, and is extremely significant for patients with CML in Brazil. It is necessary to establish a discussion focused on the creation of a code with suitable nomenclature for the test in SIGTAP,11 and make what is recommended by the disease PCDT a reality for patients.
Panel of specialists: crucial recommendations for- 1)
Molecular monitoring should be part of the integral treatment of patients with CML to reduce the chances of disease progression and costs to the health system;
- 2)
The government should put into practice what is provided in the PCDT of Chronic Myeloid Leukemia in Brazil: performing the monitoring of the molecular response via quantitative PCR;
- 3)
The government should create a code with adequate nomenclature and reimbursement value in SIGTAP, so that the test is carried out and covered by the public healthcare network, as it is contained in the PCDT of the disease and the existing APAC does not cover the operational costs for its performance;
- 4)
Patients with chronic phase CML should perform a quantitative PCR every 3 months and, after reaching the MMR, should perform the examination every 6 months, as recommended by international guidelines. The PCDT should be updated based on international guidelines regarding PCR monitoring frequency;
- 5)
Patients should be monitored in reference laboratories that are standardized according to the international scale;
- 6)
The laboratories that are within the reference public centers could absorb all the test demand in Brazil, and other centers could be qualified through an ABHH accreditation;
- 7)
Adequate molecular monitoring may allow some patients to stop taking drugs and self-financing the molecular test for all SUS patients
This study was funded by Novartis Brazil.
Conflicts of interestThe authors declare no conflicts of interest.