The etiology of stroke, a severe complication of sickle cell anemia, involves inflammatory processes. However, the pathogenetic mechanisms are unknown. The aim of this study was to evaluate the influence of interleukin-10 polymorphisms and haplotypes on the risk of acute cerebral ischemia and high-risk transcranial Doppler in 395 children with sickle cell anemia from the state of Minas Gerais, Brazil.
MethodsInterleukin-10 haplotypes were determined by polymerase chain reaction-restriction fragment length polymorphism and sequencing. The outcomes studied were acute cerebral ischemia and high-risk transcranial Doppler. Clinical data were retrieved from the children's records.
ResultsThere was no statistically significant difference in the frequencies of polymorphisms and haplotypes between children with and without acute cerebral ischemia or children with or without high-risk transcranial Doppler. These data are consistent with a previous report that showed an absence of association between interleukin-10 plasma levels and high-risk transcranial Doppler velocity in children with sickle cell anemia.
ConclusionInterleukin-10 haplotypes were not associated with the risk of acute cerebral ischemia or high-risk transcranial Doppler velocity in children with sickle cell anemia from the state of Minas Gerais, Brazil.
Cerebrovascular disease is a severe complication of sickle cell anemia (SCA), affecting almost one-half of children with SCA by the age of 14.1 The phenotype of SCA is extremely variable, and stroke occurs in 11% of under 20-year-old individuals without preemptive treatment.2 Despite the fact that transcranial Doppler (TCD) ultrasonography is very useful,3 there is a need to develop more accurate prognostic tools to identify children who are at the highest risk of developing a stroke.4 Inflammatory processes play an important role in the development of occlusive disease at the circle of Willis5 and are clearly involved in the etiology of strokes in children with SCA.6 Polymorphisms in cytokine or cytokine receptor genes have been shown to modulate the occurrence of stroke in children with SCA.7–9 Interleukin-10 (soluble interleukin-10 – IL-10) is a vital immunoregulatory cytokine involved in both immune response and inflammation. The effects of IL-10 upon inflammation include inhibition of proinflammatory cytokine production, including IL-1, IL-6, IL-12, and tumor necrosis factor (TNF), as well as both types of CC and CXC inflammatory chemokines.10Interleukin 10 gene (IL10) polymorphisms in the promoter region (known as −1082 G/A, −819 T/C, and −592 A/C) form haplotypes that are linked to different expression levels of this cytokine.11 In recent years, reports have demonstrated that IL10 haplotypes are associated with many aspects of different diseases and conditions, including survival and relapse in resected non-small cell lung cancer,12 systemic lupus erythematosus,13 asthma,14 and inhibitor development in hemophilia A.15 A recent report has shown an association between reduced IL-10 levels and the frequency, type, severity, and duration of vaso-occlusive crises in children with SCA,16 suggesting a possible influence of IL-10 on the pathophysiology of stroke. Stroke in children with SCA has been associated with a proinflammatory state characterized by recruitment of white blood cells to the endothelial lesion, microvascular obstruction and ischemia, intimal hyperplasia, fibrosis, and finally, occlusion.17 Based on these data, we hypothesized that high IL-10 production haplotypes could decrease the inflammatory state and, consequently, reduce the occurrence of stroke in children with SCA.
MethodsThis was a retrospective cohort study that included 395 children with SCA from the state of Minas Gerais in Brazil. Children who were born between January 1999 and December 2008 and followed-up at Fundação Hemominas in Belo Horizonte until June 2015 were recruited from a newborn cohort of 472 subjects with an Hb FS electrophoretic pattern. Of these 472 children, 77 (16.3%) were excluded: 32 with the Hb S/beta-thalassemia genotype, 13 with Hb S/hereditary persistence of fetal hemoglobin, one child with a hemorrhagic stroke, and 31 who could not be reached during the study or did not consent to participate. The study protocol was approved by the individual institutional review boards (Fundação Hemominas #295 and Universidade Federal de Minas Gerais #154/11). Furthermore, written informed consent was obtained from parents or guardians, and children's assent was obtained, when appropriate. The study was conducted in accordance with the guidelines of the Declaration of Helsinki.
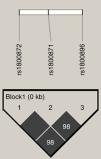
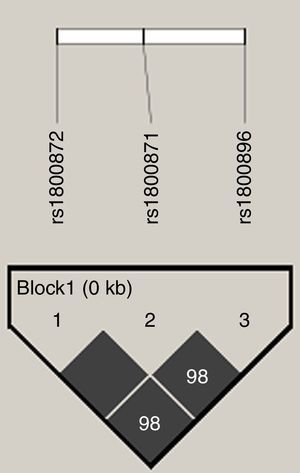
Genomic DNA extraction from blood samples was carried out using a commercial kit (QIAamp, DNA Blood Mini Kit; Qiagen, Hilden, Germany). To confirm the presence of SCA, the βS allele genotype of all children was confirmed by polymerase chain reaction-restriction fragment length polymorphism (PCR–RFLP) analysis as previously described.18IL10 promoter polymorphisms, c.−627 A>C (rs1800872; known as −592 A>C), c.−854 T>C (rs1800871; known as −819 T>C), and c.−1117 A>G (rs1800896; known as −1082 A>G), were genotyped by PCR–RFLP as previously described.19,20 At least 5% of the samples were randomly selected for DNA sequencing to confirm PCR–RFLP results. DNA sequencing was carried out in an ABI Prism 3130 Analyzer (Applied Biosystems; Foster City, CA, USA), using standard protocols. Due to complete linkage disequilibrium (LD) between rs1800872 (−592 A>C) and rs1800871 (−819 T>C) polymorphisms,21 only 70 children were genotyped for the rs1800871 polymorphism and in all, the linkage between those two polymorphisms was detected. Haplotypes of the single nucleotide polymorphisms (SNPs) were coded in the following order: rs1800896, rs1800871, and rs1800872 (for example, GCC).
Two outcomes were analyzed: acute cerebral ischemia and high-risk TCD. Acute cerebral ischemia was defined as a neurological deficit lasting more than 24h (stroke) or history of transient ischemic attack (TIA).2 The diagnosis of stroke was confirmed by imaging assessments in all cases; magnetic resonance angiography was used in two (8.7%) children, computed tomography in 12 (52.2%) children, and both in nine (39.1%). Intracranial hemorrhage was not included in the category of stroke. High-risk TCD was defined as a time-averaged mean of the maximum velocity (TAMMX) ≥200cm/s in the internal carotid or middle cerebral artery as originally defined by the STOP investigators.22 Children who had suffered acute cerebral ischemia as well as those for whom TCD screening was inadequate were excluded from the second analysis. For individuals who underwent multiple TCD tests, only the most recent was included in the analysis (High-risk TCD as outcome). For children undergoing chronic transfusion therapy, treatment with hydroxyurea, or bone marrow transplantation, the result of the last TCD exam prior to the initiation of therapy was included in the analysis.
Statistical analysisNominal variables are expressed as percentages. Univariate association between outcomes and single polymorphisms or haplotypes was evaluated using two-tailed chi-square or Fisher's exact test. Initially, the influence of each polymorphism alone was verified. Subsequently, only children with the homozygous form of each haplotype (GCC/GCC, ACC/ACC, or ATA/ATA) were tested. Finally, children were grouped into haplotypes defined as high (GCC/GCC), intermediate (GCC/ACC and GCC/ATA) or low (ACC/ACC, ATA/ATA, and ATA/ACC) levels of IL-10 production.23 The cumulative probability of outcomes was estimated using the Kaplan–Meier method [function (1-Survival)] and the log rank test was used to compare different subgroups. Birth date determined entry into the study program. Observations were censored in the event of a patient receiving chronic prophylactic blood transfusions or hydroxyurea, a patient undergoing bone marrow transplantation, death from causes unrelated to outcomes, or no outcome event until June 2015 (end of study).
Statistical significance was defined as p-values ≤0.05. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) v. 17.0 software (SPSS Inc., Chicago, IL, USA). Additionally, pairwise LD between SNPs was evaluated using the software HaploView (version 4.2; http://www.broad.mit.edu/mpg/haploview/) and expressed as D′ and R2. The haplotype block structures were calculated using the HaploView, according to the algorithm previously described.24 In each association test, a total of 1000 random permutations were performed to internally validate the association between haplotypes and analyzed outcomes.
ResultsCharacteristics of children enrolled in this study were described in detail elsewhere.25 Briefly, mean follow-up period was 9.04 years (1.32–15.74), providing 3572 patient-years. Twenty-six (6.6%; 95% CI: 4.14–9.03) children had acute cerebral ischemia; 19 (4.8%) had a stroke, three (0.7%) had TIA, and four (1%) had both stroke and TIA. Twenty-nine (8.6%; 95% CI: 5.59–11.57) out of 338 children had high-risk TCD; 57/395 (14.4%) children had acute cerebral ischemia or inadequate TCD and were excluded from the analysis for high-risk TCD. The cumulative probability of acute cerebral ischemia by the age of 8.0 years was 7.4% (95% CI: 4.66%–10.14%), and the cumulative probability of high-risk TCD by the age of 11.5 years was 14.2% (95% CI: 8.91%–19.49%).
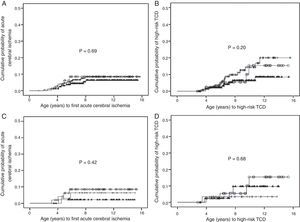
An analysis of IL10 haplotypes in this cohort showed that 99 (25.1%) children were GCC/ATA, 85 (21.5%) ACC/ATA, 79 (20%) GCC/ACC, 50 (12.7%) GCC/GCC, 47 (11.9%) ATA/ATA, and 34 (8.6%) were ACC/ACC. One (0.3%) child had an uncommon heterozygous haplotype (GCC/GTA). The SNPs were in high LD (Figure 1). There was no significant difference in the frequencies of polymorphisms and haplotypes between children with and without acute cerebral ischemia (Table 1). Similar results were obtained between children with and without high-risk TCD (Table 2). The cumulative probabilities of acute cerebral ischemia or high-risk TCD for IL-10 expression haplotype groups were not significantly different (Figure 2A and B, respectively). Similar results were obtained when only children that were homozygous for each haplotype were tested (Figure 2C and D). Likewise, HaploView analysis (haplotype associations and permutation tests) showed no association between haplotypes and outcomes (data not shown).
Linkage disequilibrium (LD) plot of the three single nucleotide polymorphisms (SNPs) in the IL10 promoter region gene. LD plot, each square (with D′ values written within the box) represents a pairwise LD relationship between the two SNPs. White, D′<1 and logarithm of the odds ratio (LOD) <2; gray, D′<1 and LOD≥2; black, D′=1 and LOD≥2.
IL10 polymorphisms and haplotypes of sickle cell anemia children with and without acute cerebral ischemia.
| Acute cerebral ischemia | ||||||
|---|---|---|---|---|---|---|
| Polymorphism | Yes (n=26) | No (n=369) | Total (n=395f) | p-Value | Odds ratio | 95% CI |
| IL10 rs1800896 (A or G)e | ||||||
| AA | 9 (5.6%) | 152 (94.4%) | 161 (100%) | 0.503a | ||
| Ag | 13 (7.1%) | 170 (92.9%) | 183 (100%) | 0.544b,c | 1.32 | 0.58–3.05 |
| gg | 4 (7.8%) | 47 (92.2%) | 51 (100%) | 0.704b,d | 1.25 | 0.41–3.77 |
| IL10 rs1800871 (C or T)e | ||||||
| CC | 12 (7.4%) | 150 (92.6%) | 162 (100%) | 0.297a | ||
| Ct | 13 (7.0%) | 173 (93%) | 186 (100%) | 0.681b,c | 0.80 | 0.36–1.78 |
| tt | 1 (2.1%) | 46 (97.9%) | 47 (100%) | 0.342b,d | 0.28 | 0.04–2.12 |
| IL10 rs1800872 (A or C)e | ||||||
| CC | 12 (7.4%) | 150 (92.6%) | 162 (100%) | 0.297a | ||
| Ca | 13 (7%) | 173 (93%) | 186 (100%) | 0.681b,c | 0.80 | 0.36–1.78 |
| aa | 1 (2.1%) | 46 (97.9%) | 47 (100%) | 0.342b,d | 0.28 | 0.04–2.12 |
| IL10 haplotypes | ||||||
| High | 4 (8%) | 46 (92%) | 50 (100%) | 0.428a | ||
| Intermediate | 13 (7.3%) | 165 (92.7%) | 178 (100%) | |||
| Low | 9 (4.8%) | 157 (93.4%) | 166 (100%) | |||
95% CI: 95% confidence interval.
IL10 polymorphisms and haplotypes among sickle cell anemia children with and without high-risk transcranial Doppler (TCD).
| High-risk TCD | ||||||
|---|---|---|---|---|---|---|
| Polymorphism | Yes (n=29) | No (n=309) | Total (n=338f) | p-Value | Odds ratio | 95% CI |
| IL10 rs1800896 (A or G)e | ||||||
| AA | 7 (5.1%) | 130 (94.9%) | 137 (100%) | 0.151a | ||
| Ag | 18 (11.5%) | 139 (88.5%) | 157 (100%) | 0.075b,c | 2.28 | 0.95–5.50 |
| gg | 4 (9.1%) | 40 (9.1%) | 44 (100%) | 0.78b,d | 1.08 | 0.36–3.25 |
| IL10 rs1800871 (C or T)e | ||||||
| CC | 15 (10.7%) | 125 (89.3%) | 140 (100%) | 0.311a | ||
| Ct | 11 (7.0%) | 146 (93%) | 157 (100%) | 0.244b,c | 0.63 | 0.30–1.36 |
| tt | 3 (7.3%) | 38 (92.7%) | 41 (100%) | 1.00b,d | 0.82 | 0.24–2.85 |
| IL10 rs1800872 (A or C)e | ||||||
| CC | 15 (10.7%) | 125 (89.3%) | 140 (100%) | 0.311a | ||
| Ca | 11 (7.0%) | 146 (93%) | 157 (100%) | 0.244b,c | 0.63 | 0.30–1.36 |
| aa | 3 (7.3%) | 38 (92.7%) | 41 (100%) | 1.00b,d | 0.82 | 0.24–2.85 |
| IL10 haplotypes | ||||||
| High | 4 (9.3%) | 39 (90.7%) | 43 (100%) | 0.198a | ||
| Intermediate | 17 (11.2%) | 135 (88.8%) | 152 (100%) | |||
| Low | 8 (5.6%) | 134 (94.4%) | 142 (100%) | |||
95% CI: 95% confidence interval.
Cumulative probability of cerebrovascular complications according to IL10 expression haplotypes. (A) Cumulative probability of acute cerebral ischemia in children with Hb SS according to IL10 haplotypes linked with IL10 expression. (○) high (n=50); (+) intermediate (n=178); and (▴) low IL10 expression (n=166; p-value=0.69). (B) Cumulative probability of high-risk transcranial Doppler in children with Hb SS according to IL10 haplotypes linked with IL10 expression. (○) high (n=43); (+) intermediate (n=152); and (▴) low IL10 expression (n=142; p-value=0.20). (C) Cumulative probability of acute cerebral ischemia in children with sickle cell anemia according to homozygous IL10 haplotypes. (○) GCC/GCC (n=50); (+) ACC/ACC (n=34); and (▴) ATA/ATA (n=47) genotypes (p-value=0.42). (D) Cumulative probability of high-risk transcranial Doppler in children with Hb SS according to homozygous IL10 haplotypes. (○) GCC/GCC (n=43); (+) ACC/ACC (n=29); and (▴) ATA/ATA (n=41) genotypes (p-value=0.68).
This is the first report to evaluate the influence of IL10 haplotypes upon the risk of cerebrovascular disease in children with SCA. Contrary to our initial hypothesis, this study showed no association between IL10 haplotypes and acute cerebral ischemia or high-risk TCD in children with SCA.
Substantial advances in the past years have established the natural history of stroke in individuals with SCA2 and have enabled early identification and treatment of children who are at the highest risk.22 However, the pathophysiology of ischemic stroke is not completely understood. Many candidate genes and polymorphisms have been associated with stroke in children with SCA.8,9,26–28 The combination of the TNFA −308 GG genotype and IL4R 503P variant was significantly associated with a predisposition for stroke in large vessels, determined by the analyses of children in a newborn cohort as part of the Cooperative Study of Sickle Cell Disease (CSSCD).9 The rs284875 polymorphism located in the TGFBR3 gene was associated27 and validated8 as a risk factor for stroke development in individuals with SCA. Recently, an association between the TNFA −308A allele and stroke in children with SCA was proposed.7
The overall frequency of IL10 haplotypes found in this study was similar to that reported in a recent study conducted in adult individuals with SCA from the northeastern region of Brazil.29 The present study found slightly higher frequencies of haplotypes associated with high and intermediate IL10 expressions.
Polymorphisms in cytokine or cytokine receptor genes may either upregulate or downregulate cytokine levels, and might play an important role in the physiopathology of some diseases. The results of this study indicate that promoter polymorphisms in the IL10 gene are not associated to susceptibility for acute cerebral ischemia or high-risk TCD in children with SCA. These data are consistent with a previous report from a cross-sectional nested prospective study that showed an absence of association between IL-10 plasma levels and high-risk TCD velocity in children with SCA.30 Thus, the IL-10 signaling pathway might not be a viable target for pharmacological interventions to prevent or treat stroke in SCA.
Although not statistically significant, survival analysis (Figure 2) suggested a trend toward acute cerebral ischemia protection in children carrying the haplotypes linked with low IL10 expression. However, this finding differs from previous published studies, associating low IL-10 levels with vaso-occlusive crises,16 osteomyelitis,31 and acute chest syndrome29 in patients with SCA. Furthermore, this contradicts a previous study showing that a lower serum IL-10 level is associated with ischemic stroke in the general population.32
Due to the relatively small number of acute cerebral ischemia and high-risk TCD events, the statistical power in the present study may have been insufficient to robustly analyze the association of IL10 polymorphisms and haplotypes with the risk of these events. Additionally, the use of medical records as the sole source of retrospective data may have placed further limitations on the possible conclusions of this study. Furthermore, confounding factors may not have been recognized, such as silent infarcts, which is one important risk factor for stroke in children with SCA.33,34
ConclusionThese results do not support the hypothesis that IL10 haplotypes are associated with the risk of acute cerebral ischemia or high-risk TCD in children with SCA. Further larger scale studies are warranted to confirm the absence of any association between IL10 haplotypes and stroke susceptibility in children with SCA.
FundingSupported by grants from the “Fundação Hemominas” (absence of grant number), “Núcleo de Ações e Pesquisa em Apoio Diagnóstico” (Nupad-UFMG; absence of grant number), “Fundação de Amparo à Pesquisa de Minas Gerais” (FAPEMIG; grant # PPM-00266-13), “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq; grant # 304530/2011-5), and “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES; absence of grant number).
Conflicts of interestThe authors declare no conflicts of interest.
The authors acknowledge all subjects and parents for their cooperation in the study. The authors also thank the Fundação Hemominas, Núcleo de Ações e Pesquisa em Apoio Diagnóstico (Nupad-UFMG), Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG; grant # PPM-00266-13), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant # 304530/2011-5), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for their financial support.