The complete blood cell count (CBC) is a crucial test for diagnosing and managing of a variety of hematological disorders, provided that a high degree of quality can be assured throughout the testing process. Although the modern hematology analyzers are now capable to generate rapid and accurate results in most clinical circumstances, spurious results related either to white blood cells (WBC) or other parameters of CBC may occur, then leading to further unnecessary investigations and/or inappropriate clinical management.1 Several causes of spuriously high and low WBC counts have been reported, thus including platelets aggregate and large platelets, presence of nucleated red blood cells, red blood cells resistant to lysis, cryoglobulins, cryofibrinogen, lipidic droplets, microorganism, embolism of adipose tissue. Since WBC aggregates have been occasionally reported as causes of spuriously low test results,1 we describe here the case of spurious WBC count observed with the hematology analyzer Sysmex XN 9000.
Case reportIn September 2019 a 70-year-old male individual underwent routine blood testing before elective surgical procedure for abdominal hernia. The examinations included CBC, routine coagulation tests (prothrombin time and activated partial thromboplastin time), and clinical chemistry (electrolytes, creatinine, glucose, total protein, urea, C-reactive protein, GAMMA-GLUTAMYLTRANSFERASE, ALANINE AMINOTRANSFERASE, ASPARTATE AMINOTRANSFERASE, TOTAL BILIRUBIN AND LACTATE DEHYDROGENASE). With the exception of gamma-glutamyltransferase, wich was slightly elevated, all other biochemical parameters were within the reference ranges.
The past medical history revealed that the patient underwent neurosurgery for meningioma in 2010 and ears, nose, and throat (ENT) surgery for neoformation of vocal cords in 2012. He was also diagnosed as having liver cirrhosis, alcohol-related (Child-Pugh Classification A), along with arterial hypertension, and arthrosis.
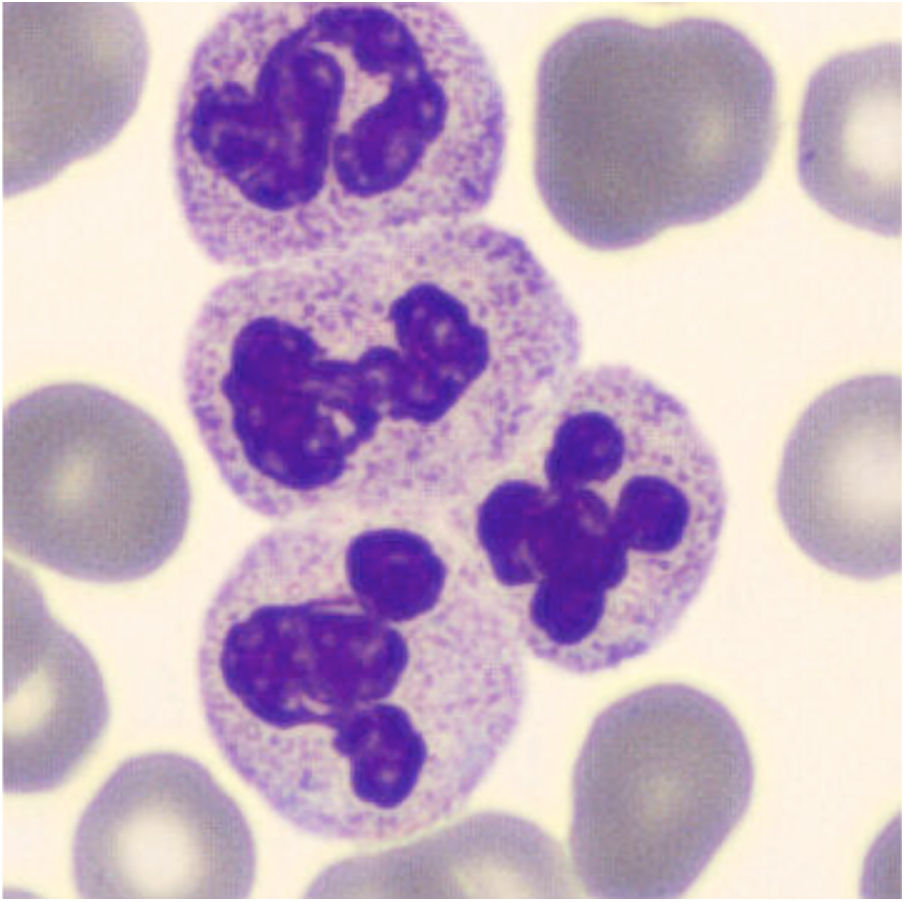
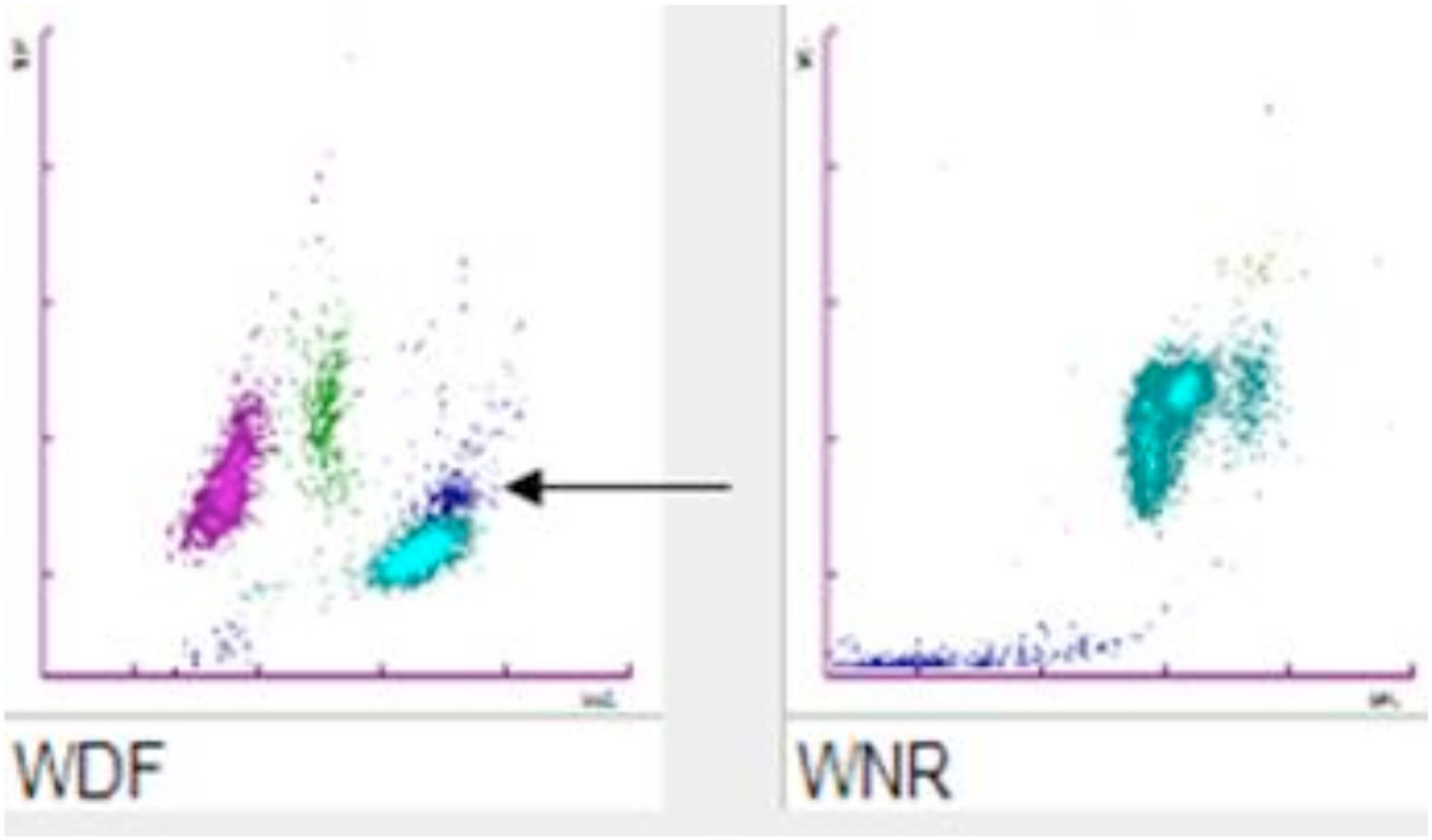
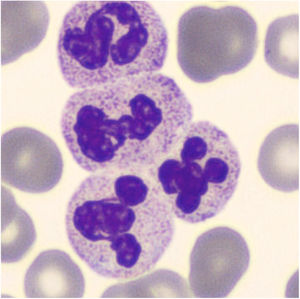
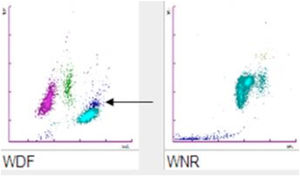
For CBC blood had been collected with a 21-gauge needle into a blood tube containing dipotassium ethylenediaminetetraacetate (K2EDTA) (Vacuette, Greiner Bio-One International©). Within 30min after blood collection, the CBC was performed on a Sysmex XN analyzer (Sysmex©, Kobe, Japan). The hematology analyzer XN enumerates and classifies blood cells with a combination of direct current detection, by using flow cytometry technology. More specifically, cell enumeration is based on semiconductor laser counting, and cells are then classified by irradiation with a 633nm laser beam and analysis of forward scattered light, side scattered light and side fluorescent light. The three signals are integrated to differentiate and count WBC, nucleated red blood cells (NRBC), reticulocytes and platelets, as well as for detecting abnormal and immature cells.2 The performance of the Sysmex XN-series has been extensively evaluated by Seo et al., as described elsewhere.3 All parameters of CBC were within the reference ranges except for a minimal leukocytosis of 10.9×109/L (reference range, 3.6–10.5×109/L) with a differential leukocyte count of 57.2% neutrophils (6.3×109/L), 35.2% lymphocytes (3.8×109/L), 7.1% monocytes (0.8×109/L), 0.3% eosinophils and 0.2% basophils. Nevertheless, an analyzer flag accompanied the data, suggestive for the presence of immature granulocytes (alarm message: “IG present”, with a count of 5.4% of immature granulocytes). The “atypical” cloud observed in the WDF (White Cell Differential) scattergram was responsible for the generation of the alert “IG” message. Due to the presence of the flag “IG presence” a blood film was prepared. The microscopic observation of the blood film showed normal erythrocyte and platelet morphology, and no immature leukocyte forms were seen. Nevertheless, some leukocyte aggregates, mostly composed of neutrophils, were detected. Microscopic observation at 400 magnifications revealed the presence of one or two aggregates in about 50% of the 100 observed fields. These aggregates were found to be composed of a variable number of neutrophils (from 2 to 5–10, in rare cases even more) (Fig. 1), some of which containing also one or two monocytes. The scattergram patterns provided by the XN analyzer are shown in Fig. 2. Interestingly, in the area corresponding to the neutrophil region in the scattergram provided by the WDF channel, a small cloud was observed that could have been generated by cells with a nucleic acid content apparently higher and which was interpreted as being small households. In the present case, this area may have been produced by little aggregates. Although the WBC count generated by the WNR channel is that finally reported by the analyzer, the count generated by the WDF channel can also be accessed through a dedicated menu. In our case report the two counts were similar (10.9 and 10 WBC×109/L in the WNR and WDF channels, respectively).
The presence of these aggregates prompted us to investigate whether a potential underestimation of WBC count may have occurred, since the manufacturer claims that the analyzer may erroneously report a low white blood cell count when leukocyte aggregates are present.4 Then, we performed another WBC count on the same sample using a flow cytometer (Navios Flow Cytometer© - Beckman Coulter, CA, USA) based on the principle of light scatter and intensity of fluorescence signal (employing propidium iodide, a DNA intercalating agent, for cell count.7 The fluorescent plot provided by the instrument showed unexpected fluctuations of fluorescence, which were positively correlated with the polyploid DNA content, corresponding to passage of leukocyte aggregates within the detection chamber with a WBC count of 10,130/μL.
. Although EDTA may induce leukocyte aggregation, this shall be considered a relatively rare finding compared to the well-known phenomenon of EDTA-induced thrombocytopenia.9 The total WBC count in our citrate sample was found to be 10.0×109/L. Interestingly, the same type of leukocyte aggregates was observed in this sample by blood smear revision. We also repeated the CBC and blood smear revision after incubation at 37°C for 2h and for 24h without observing any significant difference for both the WBC count and the presence of aggregates. After 3 days the WBC count was still 9.8×109/L and identical aggregates were seen on the blood smear. The investigation was based on pre-existing samples after making the sample anonymous and in accordance with the Declaration of Helsinki and under the terms of all relevant local legislations.
DiscussionThe phenomenon of leukocyte agglutination in peripheral blood is reported as a rare and possible transient event.1 The scientific literature describes some cases associated with cancers, severe infections, autoimmune disorders or hepatic diseases.8 Although the phenomenon of EDTA-induced platelet agglutination is relatively frequent,9 some cases of in vitro leukocyte aggregation have also been reported. Spurious WBC counts due EDTA -induced leukoagglutinins has been reported in association with cirrhosis, infections, autoimmune diseases, uremia, malignancies and immunosuppression8 Anand M et al. concluded that the leukocyte aggregation in vitro is a time-dependent phenomenon.10 In our case the larger aggregates were overlooked by the analyzer, thus leading to underestimating the WBC count.1,4 Instead, the scattergram generated by the WNR channel was normal. In WDF channel, WBC are counted and differentiated, whilst abnormal cells (e.g., immature WBC and atypical lymphocytes) are detected with a dedicated reagent. The WNR channel counts WBC and perform differential counting of basophils and NRBC with a different reagent.4 In this case specimen was stored at room temperature for 24h, confirming the presence of the leukocyte aggregates. Since the presence of cold agglutinins reacting with WBC antigens is a similar phenomenon,1 we repeated the CBC and the blood film after incubation at 37°C to rule out this possibility, but we failed to observe any difference. In a previous article, Tantanate described an incorrect WBC count using an XN analyzer, showing that the WBC count was very low in the WNR channel but was normal in the WDF channel as well as using a different analyzer and after blood smear revision. It was hence concluded that these differences were due to the use of different reagents in the two channels (i.e., more acid in the WNR channel compared to the WDF channel). The more acid reagent may cause the lysis of fragile WBC, especially in the presence of abnormal WBC or in patients receiving cytotoxic drugs.6 The mechanism leading to leukocyte aggregation has not been entirely elucidated, though more frequently attributed to EDTA.1 Unlike the case earlier published by Sultan and Irfan8 who report on a pregnant woman with spurious low WBC count due to EDTA- induced agglutination (no leukocyte aggregates could be observed in a paired citrate-plasma sample), the leukocyte aggregates in our case were also present in the citrate sample.
Interestingly, Hoffman reported that EDTA-induced leukoagglutination may be resolved by adding kanamycin sulphate to the sample but, as earlier discussed, agglutination was not probably caused by EDTA in our case.10 Unfortunately, the patient did not show up for further evaluation, consequently we were unable to perform further evaluations as the collection of a blood sample use of Anticoagulant Citrate Dextrose Solution-A (ACD-A) that in some cases is preferred because this phenomenon cannot be always eliminated using citrate as an additive. Although we cannot provide a definitive explanation on the real nature of these aggregates, it is important that laboratory professionals acknowledge the presence of leukocyte aggregates by correctly interpreting tests results, as well as the appearance of abnormal patters in the scattergrams and flag, as in this case the alert “IG” message, of their hematological analyzers.