MF is a myeloproliferative neoplasm (MPN) in which proinflammatory cytokines derived from malignant cells drive bone marrow fibrosis typical of the disease. Patients with this condition can progress to bone marrow failure, with extramedullary hematopoiesis in the liver and spleen.1 Indeed, MPNs represent one of the most common underlying reasons for patients requiring liver transplantation.2 Definitive treatment of MF requires allogenic hematopoietic stem cell transplant (alloHSCT), which has been shown to provide an overall survival benefit in patients with disease classified as Intermediate-1 or greater by the Dynamic International Prognostic Scoring System (DIPSS). However, risks of alloHSCT may outweigh potential benefits in patients with less severe disease.3 Due to a paucity of literature, it is currently unclear how prior liver transplantation impacts this risk stratification. Clinicians seeking to practice evidence-based care would benefit from further descriptions of outcomes following sequential transplantations. To this end, we provide a description of our experience using alloHSCT as a treatment for MF in a patient who had undergone liver transplantation 15 years prior. This case provides valuable insights into the feasibility of such an approach.
Case reportA 39-year-old man presented with splenomegaly and an extensive history of thrombosis. He had previously undergone a liver transplantation from a deceased (unrelated) donor in 2003 due to Budd-Chiari syndrome in the context of an undiagnosed hypercoagulable state. The Thrombophilia work up was negative except that the patient was heterozygous for Factor V Leiden. Mycophenolate mofetil and tacrolimus were used for immunosuppression. He had recurrent post-transplant portal hypertension and an inferior vena caval obstruction while maintained on dalteparin.
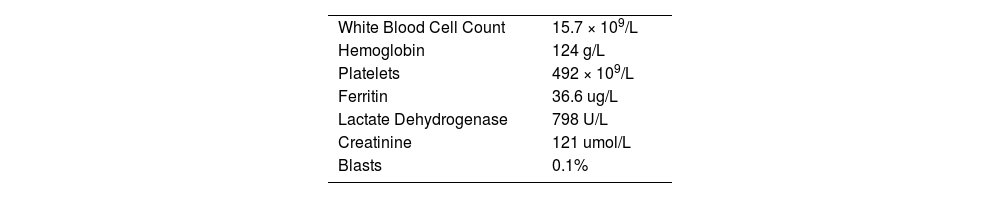
On presentation in 2018, the patient's spleen measured 40 cm and appeared to have grown insidiously since the time of his liver transplant. He reported unintentional weight loss of 30-40 kg over the same period. The patient's JAK-2 testing was positive for c.1849G>T (p. V617F) with no additional mutations found on the Oncomine Myleoid NGS panel (ThermoFischer, MA, USA).4 Blood smear showed leukoerythroblastosis with tear drops. Bone marrow biopsy confirmed the diagnosis of primary myelofibrosis, MF-3 severity, with his DIPSS score 3 (Intermediate Risk) and DIPSS-Plus score 4 (High risk). Key investigations at time of diagnosis are found in Table 1.
The patient was first treated with Ruxolitinib (15mg b.i.d.), followed by splenectomy in an effort to improve the chances of successful engraftment post alloHSCT. Six months following splenectomy the patient was considered eligible, for alloHSCT. Despite numerous transfusions in the preceding year, the patient did not had evidence of iron overload prior to alloHSCT and had a ferritin of 113ug/L. The donor was a matched related donor (sibling). A reduced intensity conditioning regimen with fludarabine, busulfan, and total body irradiation (200cGy) was used and a the PBSC cell dose of 7.1 × 106 CD34+ cells/kg from PBSCs was infused.5 GVHD prophylaxis consisted of ATG on days -3, -2, and -1 (total 4.5mg/kg), continuation of prior tacrolimus, and addition of methotrexate on days +1, +3, and +6. The transplant was complicated by culture negative febrile neutropenia, WHO grade II mucositis, CMV reactivation, and C. difficile colitis. Neutrophil and platelet engraftment occured on day +16 and peripheral blood chimerism on day +60 showed 100% Myeloid, 100% B-cell lineage, and 98.5% T-cell lineage donor cells. JAK-2 testing was also negative following transplant.
Four months after his stem cell transplant, the patient developed jaundice, hepatic encephalopathy, and elevated bilirubin and AST. There was also documented cytomegalovirus (CMV) viremia. The differential for liver pathology included acute liver GVHD, underlying liver cirrhosis, and CMV reactivation. Although liver biopsy showed cirrhosis and was suggestive of late onset veno-occlusive disease, there was no evidence of acute graft versus host disease in the liver. Previous biopsy at time of splenectomy had not shown cirrhosis. He also had evidence of acute kidney injury, thought to be due to tacrolimus induced thrombotic microangiopathy. His encephalopathy improved with supportive therapy and his immunosuppression was switched to low dose sirolimus, which he continues on at present.
The patient is currently 18 months post alloHSCT. Currently his stem cell transplant has complete donor chimerism and his liver function is stable and molecular testing by NGS negative for JAK-2 mutation.
DiscussionWe describe a case of 39-year-old man with a history of liver transplantation who was newly diagnosed with MF and successfully underwent alloHSCT for the treatment of his Myelofibrosis. In patients with MF, the decision to pursue alloHSCT depends on numerous patient and donor related factors.6 It is currently unclear how prior liver transplantation impacts the risks of undergoing alloHSCT. In 2015, Basak et al. reported a retrospective analysis of patients undergoing alloHSCT following solid organ transplant (SOT). In their study the thirteen patients undergoing alloHSCT following liver transplantation performed better than those receiving stem cell transplants after kidney or heart transplantations. The incidence of liver rejection in this case-series was 15% (95% CI: 2-40) and overall survival was 51% (95% CI:16-86) at 60 months.7 While none of these patients had MF, their data supports the notion that alloHSCT can be of benefit in patients with prior liver transplantation. Jurdi et al (2020) also performed an analysis of alloHSCT following SOT, concluding this strategy could be beneficial in appropriately selected patients. Although they demonstrated superior outcomes for patients with prior kidney transplant relative to liver, their study included both allogeneic and autologous HSCTs and included only 4 patients with prior liver transplant.8 Doney et al. (2015) identified 19 patients in the literature and 5 at their institution who underwent liver transplant followed by alloHSCT. They concluded that pre-existing SOT did not necessarily adversely impact outcomes of subsequent alloHSCT.9
In 2009, Perz et al. described their experience providing alloHSCT from a single mismatch donor to a patient with polycythemia vera progressing to MF and prior deceased donor liver transplant. While this patient experienced graft versus host disease (GvHD) manifesting as scleroderma, keratoconjunctivitis sicca, continuing cachexia, and polyneuropathies, stem cell engraftment and liver function appeared well maintained at 18 months post alloHSCT.10
Liver transplant is unique amongst SOTs due to the plethora of mechanisms by which the graft can induce allo-tolerance.11 Interestingly, the large number of leukocytes present in the liver can partially populate and coexist in the context of the host immune system, a phenomenon known as microchimerism.12 It has been suggested that microchimerism following liver transplantation can not only prevent rejection of this organ by the new graft, but also facilitate subsequent stem cell engraftment in alloHSCT.10 Indeed, combined liver transplantation with infusion of donor-derived hematopoietic stem cells has been proposed as a means of increasing long-term liver acceptance in transplantation.13 It is unclear, however, how previous microchimerism would impact subsequent alloHSCT with a genetically different donor and whether this three-way chimerism would improve engraftment of the new stem cell population. Microchimerism can be assessed by usual qPCR techniques as well as STR (Single Tandem Repeats) and VNTR (Variable Number of Tandem Repeats) analysis. However, we were unable to carry this analysis as we were not privy to the HLA status of the deceased liver donor. An additional limitation to our work is that, because liver transplantation was not carried out at our center, some details of patient records surrounding this time were not available.
In conclusion, we provide a report of a patient with MF who successfully underwent alloHSCT from a related donor 15 years after having received an unrelated liver transplant. Although his initial clinical course was complicated, at present the patient's stem cell transplant remains engrafted and his liver function preserved. This supports conclusions found in the literature that prior liver transplantation need not exclude patients from subsequent hematopoietic stem cell transplantation. The immune environment established in the patient following sequential liver transplant and alloHSCT is an ongoing area of interest, in which further development of preclinical models may provide useful insights.