Intraocular lymphoma (IOL) is a rare presentation of lymphoma with a prevalence of 0.047 cases per 100,000 people.1 The IOL can be divided into primary intraocular lymphoma (PIOL), a subtype of the primary central nervous system (CNS) lymphoma, and secondary intraocular lymphoma, when it is originated from a systemic disease. PIOL can be divided into vitreoretinal, choroidal and iridal lymphomas, according to the initial presentation. Primary vitreoretinal lymphoma (PVRL) is the most prevalent subtype among primary PIOL and may involve the vitreous humor, retina, retinal pigment epithelium, and optic nerve. The Bruch's membrane located between the retinal pigment epithelium and the choroid, contributes to the hemato-retinal barrier helping to prevent PVRL systemic spread as well as intraocular involvement by primary choroidal lymphoma and secondary lymphoma cells.2 Diffuse large B-cell lymphoma (DLBCL) is the most prevalent histological type of PVRL, while extra-nodal marginal zone lymphoma (MZL) is the most common histological type of primary choroidal lymphoma.3
PVRL clinical manifestations can be initially diagnosed as vitreitis, uveitis, vasculitis, optic disc edema, retinal ischemia or hemorrhage, retinal detachment, and secondary glaucoma. On fundoscopy, it is classically observed the presence of yellow-white infiltrates in the subretinal epithelium, which increases in size with the disease progression. Blurred vision, vision loss and floaters are common eye complaints. Due to its low prevalence, similar signs and symptoms to other eye diseases coupled with difficulties in obtaining ocular specimens, PVRL is often lately diagnosed increasing the risk of extraocular involvement and worsening clinical outcome.3,4
The aim of this study was to report a case of a patient with PVRL to exemplify difficulties in diagnosis and the need of challenging decisions. We review the literature to propose a diagnostic algorithm to help ophthalmologists and hematologists in PVRL diagnosis and management.
Case reportA 61-year-old woman with hypothyroidism, asthma, and dyslipidemia came to an ophthalmological consultation in May 1, 2018. She reported a 6-month history of progressive visual loss in the right eye (OD). On examination, the conjunctiva was normal, and the cornea was clear. The biomicroscopy showed a normal anterior chamber, trophic iris, and clear lens in both eyes. On fundoscopy, the left eye was normal. In the OD, the retina was attached, the optic disc had sharp margins with cup to disc ratio of 0.3, but the macula had decreased brightness, vessel tortuosity, and multiple yellowish lesions were observed in the temporal retina and adjacent to the posterior pole. The OD retinal pigment epithelium was moderately atrophic.
OD retinal fluorescein angiography (FA) revealed a macular lesion with hypofluorescence in the early phase and hyperfluorescencein in the late phase, suggesting circulatory abnormality. OD optical coherence tomography (OCT) revealed a subretinal elevated lesion being IOL considered as a possible diagnosis (Figure 1A). A brain magnetic resonance imaging (MRI) was performed and did not show abnormalities. Computed tomography (CT) of the chest, abdomen, and pelvis did not show lymphadenopathies. Laboratory tests were requested: interferon-gamma release assay for tuberculosis was negative. Serology for Herpes Simplex Virus Type 1 and Type 2, Varicella Zoster and Cytomegalovirus were reactive for IgG and non-reactive for IgM. Anti-HIV serology and VDRL were non-reactive. The patient was referred to hematologist for evaluation. OD visual loss was the only complaint. She denied fever, night sweats or weight loss. The physical examination findings were unremarkable. The laboratory tests were Hb 16.0 g/dL (range 12-15 g/dL), white blood cell count of 10,630/µL (range 3,500-10,500/µL) with a differential of 63% segmented, 1% eosinophil, 33% lymphocytes, 3% monocytes. Platelets count 194k/µL (range 140-450k/µL). C-Reactive Protein of 1.4mg/dL (up to 3mg/dL), Erythrocyte Sedimentation Rate of 2 mm/1 hour, Lactate Dehydrogenase of 341 IU/L (range 240-480 IU/L), and Beta-2 microglobulin of 2.2 mcg/mL (up to 2.7 mcg/mL).
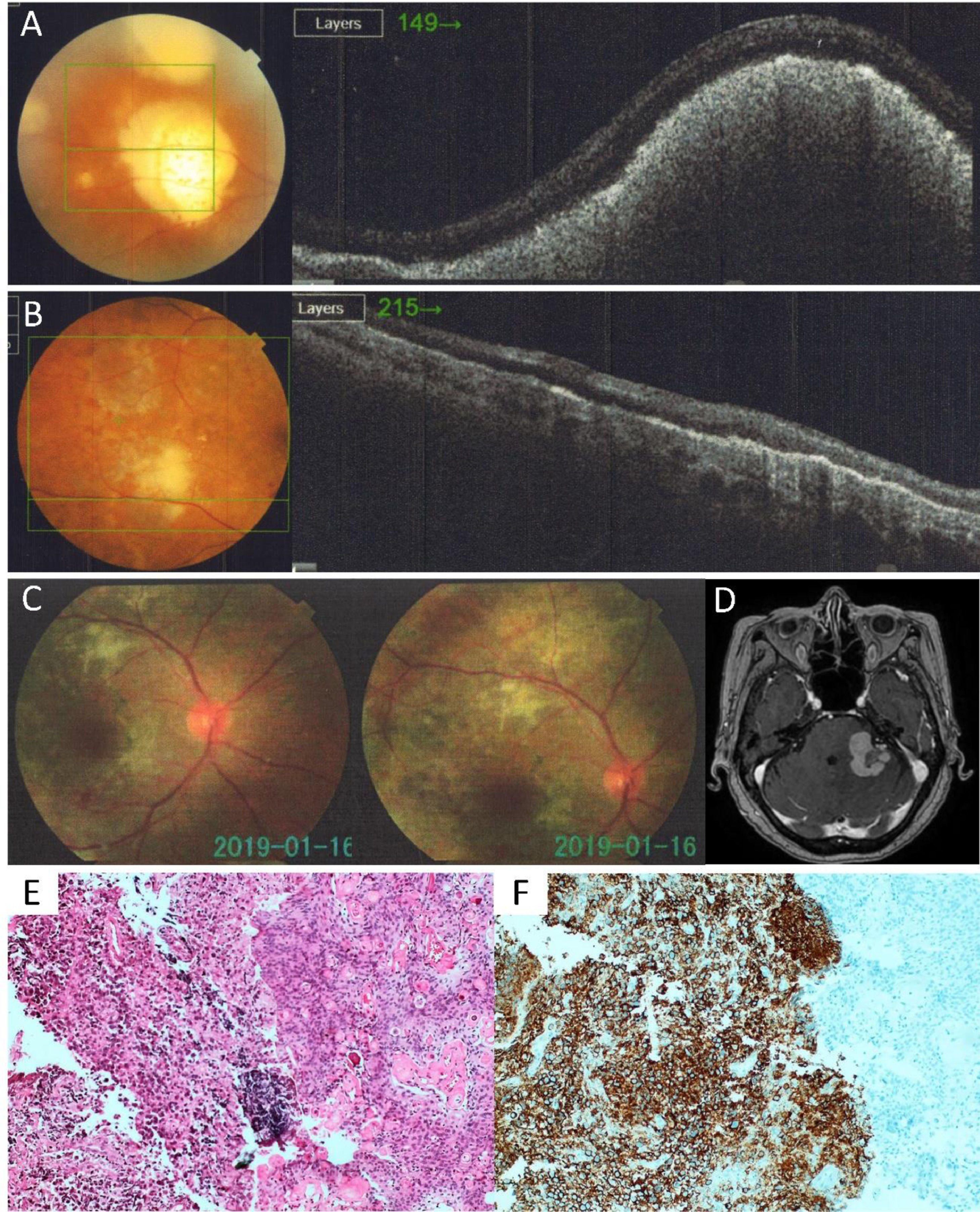
(A) OD OCT before treatment showing an elevated subretinal lesion; (B) OD OCT after treatment showing a size reduction of subretinal lesion; (C) Retinography showing a macula with decreased brightness, vessels tortuosity, retinal pigment epithelium moderately atrophic with areas of retinal hypopigmentation and isolated microhemorrhages; (D) Brain MRI with a 3 cm expansive lesion in the left cerebellar hemisphere; (E) Hematoxylin and eosin stain of the brain biopsy reveals a diffuse infiltrate of lymphocytes in the left half of the figure. The right half shows the angiomatous meningioma. (F) Immunohistochemistry positive for CD20 marker.
Oral Prednisone 60 mg daily was prescribed for two weeks without any improvement in the OD vision. One week after discontinuing prednisone, a vitreous humor specimen was obtained and sent to flow cytometry analysis. Unfortunately, the material had very few cells and no clonal lymphocyte population was identified.
In September 2018, due to progressive worsening, intravitreal injection of methotrexate 500µg was initiated weekly, with a partial improvement of the OD vision associated with a size reduction of subretinal lesion observed in a new OCT (Figure 1B). A total of nine injections of intraocular methotrexate were administrated. The OD retinography performed in January 2019 (Figure 1C) showed a macula with decreased brightness, vessels tortuosity, retinal pigment epithelium moderately atrophic with areas of retinal hypopigmentation close to the temporal arches and adjacent to the posterior pole and isolated microhemorrhages.
The persistence of the OD lesion with a partial response to intraocular chemotherapy was a key factor for deciding in favor of systemic chemotherapy for CNS lymphoma. Two high dose chemotherapy sessions with 3.5 g/m2 methotrexate over 24 hours and cytarabine 2.0 g/m2 every 12 hours for 2 days were prescribed in March and April 2019. Intrathecal chemotherapy with methotrexate 10 mg was also administrated in both sessions. The cerebrospinal fluid analysis was negative for neoplastic cells on both occasions.
In May 2019, the patient complained of dizziness, unsteady gait, and fatigue. Her clinical examination was unremarkable except for her neurological examination that showed dysthymia, presence of bilateral horizontal nystagmus, unbalance, and ataxic gait. The brain MRI showed a 3 cm expansive lesion in the left cerebellar hemisphere and left middle cerebellar peduncle, with a mass effect on the fourth ventricle and brainstem (Figure 1D). Craniotomy for biopsy of the lesion was performed in July 2019 and the result was consistent with diffuse large B-cell lymphoma associated with an angiomatous meningioma (Figure 1E). The tumor immunohistochemistry was positive for CD20 (Figure 1F), BCL-2, MUM1, Ki-67 (95%) and MYC (80%) and negative for CD10, CD3 and BCL-6.
Unfortunately, the patient's clinical and neurological condition deteriorated rapidly, and the patient died in August 2019, before radiotherapy was initiated.
DiscussionBlurred vision, vision loss and floaters might be the only complaints for patients with PVRL. An ophthalmological examination may reveal posterior uveitis, vitreitis, optic disc edema, retinal hemorrhage, secondary glaucoma, among other non-specific findings. On fundoscopy, a yellow-white infiltrates in the subretinal epithelium associated or not with vitreous opacities are suspicious findings for PVRL. These infiltrates are composed of clusters of subretinal lymphocytes in between healthy areas forming a “leopard skin” pattern, which is better characterized by FA.5 Differential diagnosis can include retinitis due to cytomegalovirus, toxoplasmosis, syphilis, tuberculosis, herpes simplex virus, HIV, sarcoidosis, bacterial endophthalmitis and ocular metastatic lesions.6 For latent tuberculosis, the interferon-gamma release assay (IGRA) is a useful exam to detect active infection, while for the other infectious diseases, serology may help to reach the final diagnosis.
Retinography, retinal OCT and retinal FA are important to characterize the aspect, the extent, and the evolution of retinal and subretinal lesions. Hyperfluorescent and hypofluorescent areas are usually observed by FA, while infiltrative or nodular lesions are seen in OCT.6 Positron emission tomography (PET-CT) and brain MRI are recommended to identify extraocular lesions suspected of lymphoma, which could be easier biopsied than the retina. CSF collection for a cytological exam is indicated if symptoms of lymphoma meningeal involvement or CNS involvement in brain MRI is present.5
Intraocular samples for the diagnosis are obtained by fine needle aspiration (FNA) or pars plana vitrectomy (PPV). FNA directed to subretinal lesions offers greater chances of obtaining material for diagnosis than PPV.7 The decision between using FNA or PPV depends on disease location and extension. The first is applied for lesions located in the retina while the second is preferred when vitreous opacities need to be cleared.3,5
Cytology, immunohistochemistry, and flow cytometry analysis of FNA samples have the potential to diagnose up to 60% of PVRL with very few false positive results. The material must be processed within a few hours of the collection due to accelerated cell degradation and apoptosis. The use of corticosteroids up to two weeks before obtaining the aspirate may induce cell apoptosis and should be avoided to increase the chance of diagnosis.1,3,6
Interleukin 10 (IL-10) and interleukin 6 (IL-6) measurements may also be performed in the vitreous humor. The elevation of IL-10 and the IL-10/IL-6 ratio greater than 1 suggest PVRL, while the elevation of IL-6 or the IL-10/IL-6 ratio less than 1 suggests inflammation.8 Dosages of interleukins have no diagnostic purpose, but if the IL-10/IL-6 ratio suggests lymphoma, it could be useful to indicate FNA or PPV procedures. It is also useful for indicating a second procedure when no cells were found previously.
The L265P oncogenic mutation in the MYD88 gene can be detected in very small volumes of both aqueous and vitreous humor by polymerase chain reaction (PCR). The sensitivity of this test was 67% and 75% for aqueous and vitreous humor, respectively, with 100% specificity for both fluids.9 If flow cytometry detects a population of CD20 positive cells in the vitreous humor but its result is inconclusive for diagnosing VRL, the detection of MYD88 L265P mutation can be applied for confirming VRL.2
If the incisional or excisional biopsy of the retina is needed, it must reach the choriocapillary portion where there is a higher chance of obtaining lymphoma cells. The indication of biopsy will depend on the location of the lesion and the results of FNA or PPV.10
Eye enucleation may be indicated if the eye is blind or painful, and no conservative treatment is possible. It is interesting to point out that eye enucleation is not only an exclusively diagnostic approach, but also a curative procedure. In retrospective analyzes of patients with IOL, enucleation was associated with increased survival.11
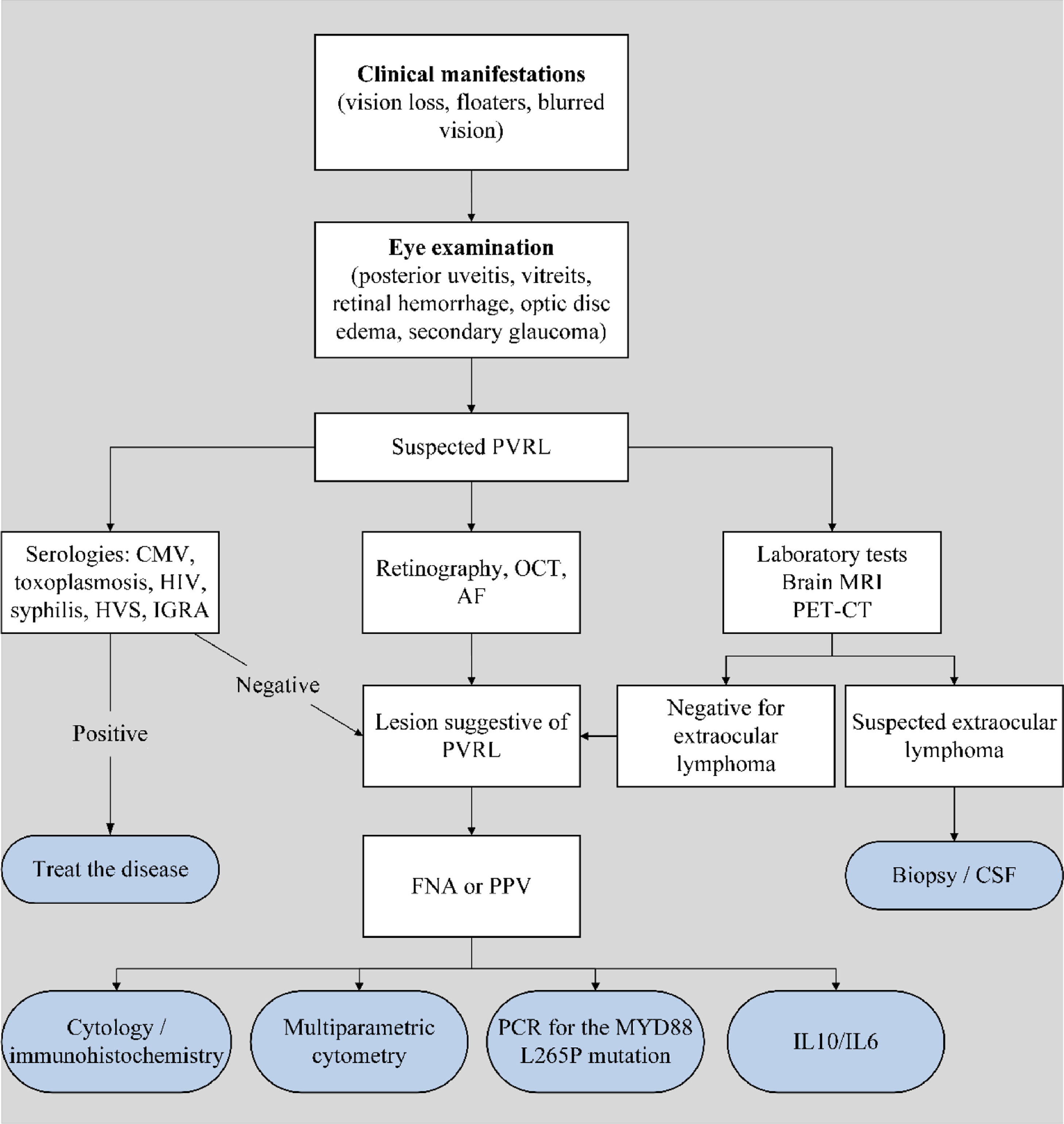
Hematologists and ophthalmologists must work closely together for proper diagnosis. Based on the reported clinical case, hematologists should support ophthalmologist in the decision for performing FNA, PPV or retinal biopsy to guarantee the proper diagnosis of lymphoma. Without a pathology report no proper treatment can be prescribed, the disease may progress locally and involve other brain structures, significantly worsening clinical prognosis. A diagnostic algorithm is suggested in Figure 2.
IOL diagnostic algorithm. (PVRL: primary vitreoretinal lymphoma; OCT: optical coherence tomography; FA: fluorescein angiography; MRI: magnetic resonance imaging; PET-CT: positron emission tomography; CMV: cytomegalovirus; HIV: human immunodeficiency virus; HVS: herpes simplex virus; IGRA: interferon-gamma release assay; CSF: cerebrospinal fluid; FNA: fine needle aspiration; PPV: pars plana vitrectomy; PCR: polymerase chain reaction; IL: interleukin).
Although PVRL is a rare disease, its clinical manifestations resemble symptoms and signals of common eye disorders. A diagnostic algorithm for PVRL is not well established. Most evidence related to PVRL is based on reports of a few selected cases and small case series. The diagnosis of PVRL in early stages, without concomitant CNS involvement, is challenging. The average time required from the onset of the symptoms until diagnosis is one year.1,2 This delay is usually caused because hematologists are not familiar with eye pathologies, while ophthalmologists are not used to the lymphoma diagnosis. It is essential that hematologists and ophthalmologists be aware of the disease and act together to reduce the time for diagnosis and improve clinical management of PVRL.