Anti-Human Leukocyte Antigen (HLA) antibodies are preformed antibodies against class I and/or class II HLA antigens that form as a result of exposure to foreign HLA antigens. Donor specific anti-HLA antibodies (DSA) in an allogeneic transplant recipient are directed against the potential donor's HLA antigens. Increasing use of partially HLA-mismatched donors allows for the possibility of DSA that increase the risk of poor graft function and graft failure.1 Risk factors for the presence of DSA include pregnancy, blood product transfusion or prior organ transplantation. The incidence is reported to be up to 43% in parous women and 12.5% in nulliparous women.2
DSA are considered to be weak or low level with phenotype panel mean fluorescence intensity (MFI) values ranging from 1000 to 3000; moderate intensity at MFI of 3000 to 5000; and strong when MFI is greater than 5000.3 When an alternate donor is not available, desensitization strategies are employed in an attempt to decrease antibody titers to levels (usually MFI <1000) that may allow successful engraftment. Desensitization strategies utilized and published in the literature include intravenous immune globulin, plasmapheresis, rituximab, bortezomib, donor HLA matched platelet infusions and staphylococcal protein A adsorption.4-7 We report a case of a patient with high titer DSA treated with the anti-CD38 monoclonal antibody daratumumab resulting in successful desensitization and engraftment.
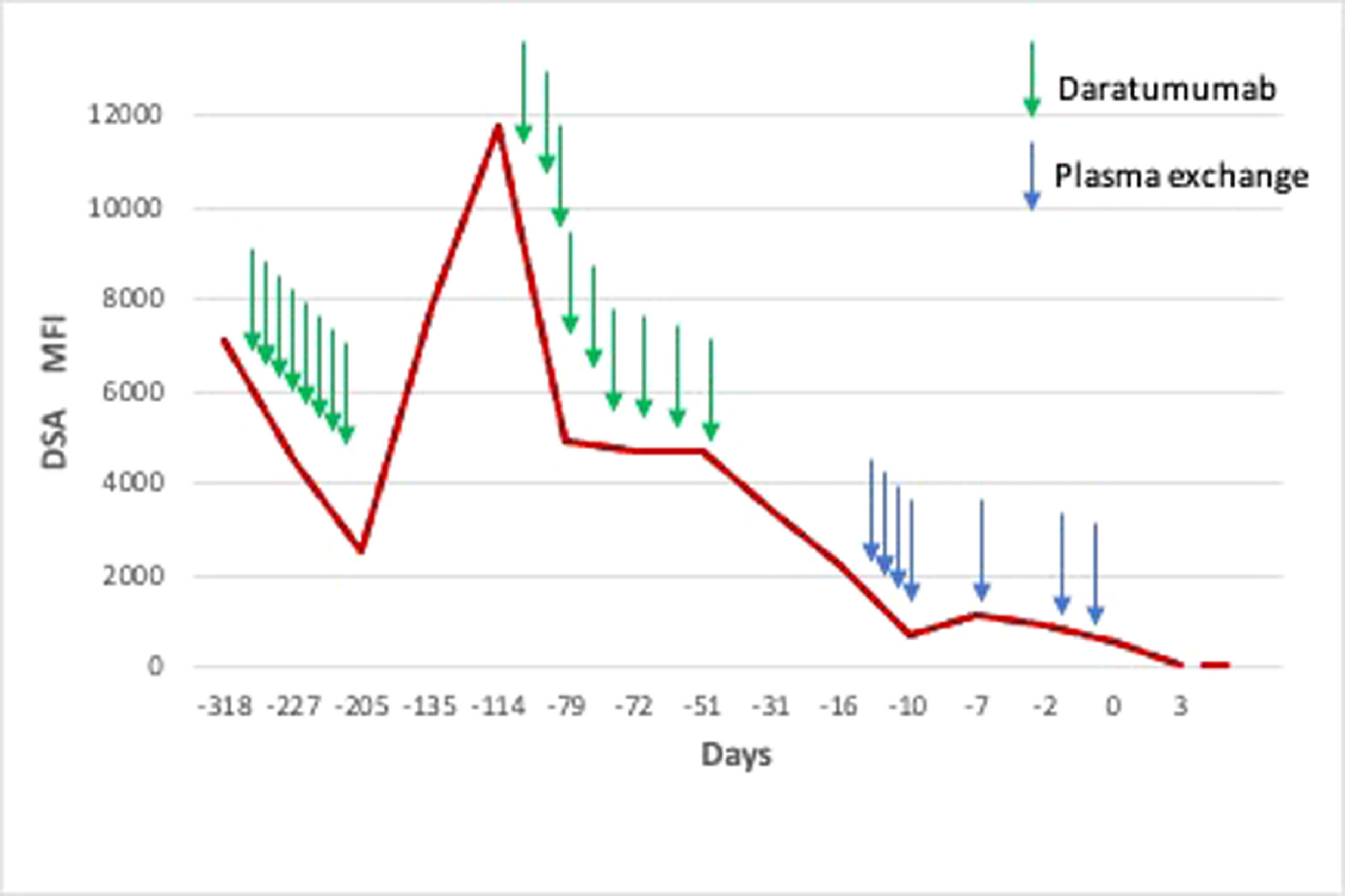
CaseA 60-year-old female with JAK2 mutated post-essential thrombocythemia myelofibrosis with a complex karyotype was referred for allogeneic stem cell transplant evaluation. Her DIPSS-plus score was intermediate-2, MIPSS70 intermediate risk and MIPSS70-plus very high risk. The patient was gravida 3, para 3. A few months prior to presentation, the patient was evaluated at another institution for transplant evaluation and two of her children were identified as potential donors. However, the patient was noted to have DSA to HLA-DQ7 with an MFI of 18,600. Desensitization was attempted and the patient receiving a dose of rituximab and underwent three sessions of therapeutic plasma exchange (TPE). DSA remained positive and transplant was put on hold. The patient transferred her care to us and a 9 out of 10 unrelated male donor was identified, also with mismatch at the HLA-DQ7 locus. DPB1 was non-permissive and no antibodies to DPB1 were present. At this time her DSA MFI was 7069. Since prior desensitization strategies had been insufficient at depleting DSA, we decided to treat the patient with daratumumab based on data in desensitization in solid organ transplantation.8 After eight weekly doses of daratumumab 16 mg/kg intravenously, the DSA decreased to MFI 2488. The transplant was delayed because of the COVID-19 pandemic and repeat titer after 12 weeks off daratumumab was consistent with a rebound increase in DSA level to MFI 11,786. We resumed desensitization with daratumumab 16 mg/kg IV weekly for nine doses in combination with bortezomib 1.3 mg/m2 SC weekly for eight doses resulting in DSA titer decrease to MFI 4921.
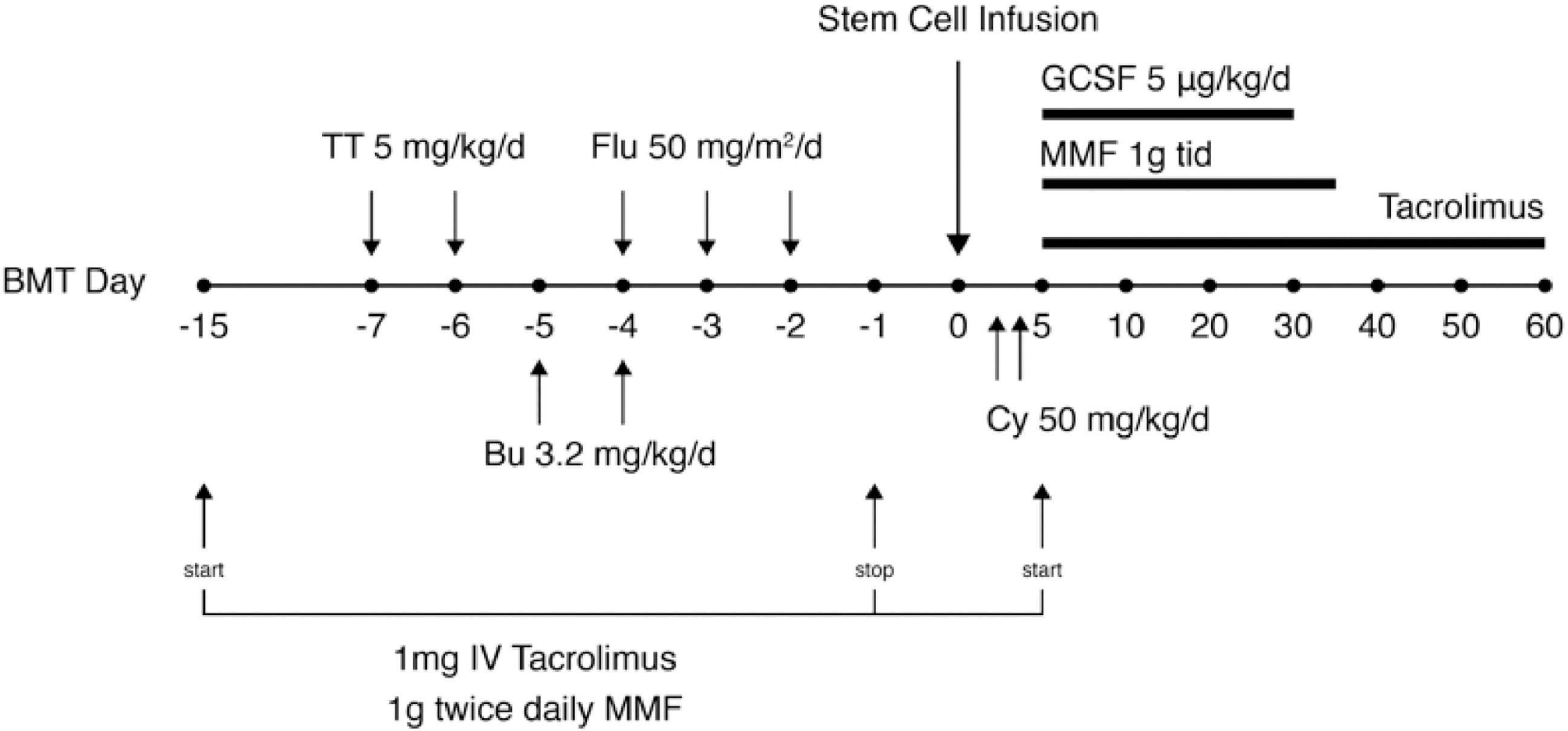
Thereafter, we followed the protocol by Gladstone et al of alternate day PP with IVIG replacement.3 PP was performed on days -15, -13, -11, -9 and-7. Additional TPE was performed on days -2 and –1 because of a small rebound in MFI on day –7 (MFI 1154 up from 669) that resulted on day –3 (Figure 1). Tacrolimus 1 mg IV and MMF 1 g twice daily were administered from days -15 to day -1. Conditioning comprised of thiotepa 5 mg/kg on days -7 and -6, busulfan 3.2 mg/kg on days -5 and -4 and fludarabine 50 mg/m2 on days -4, -3 and -2. GVHD prophylaxis comprised of tacrolimus, MMF and post-transplant cyclophosphamide 50 mg/kg on days +3 and +4 (Figure 2). DSA negativity (MFI 534) was achieved by day -1. The patient was infused peripheral blood CD34+ cells at a dose of 6.87 × 106 cell/kg. neutrophil engraftment occurred on day +25. By day +30, CD33 peripheral blood chimerism was 100% and CD3 chimerism was 100% by day +60. DSA remained negative post-transplant, however, the patient had evidence of disease relapse by day +103 when bone marrow FISH showed 9% of cells showing deletion (one copy) of D13S319 (13q14.3) locus which was present in 81% cells prior to transplant. The patient was started on azacitidine and after six cycles has no evidence of disease and 100% Y by FISH at the time of this publication.
DiscussionAnti-HLA donor specific antibodies pose a hurdle to successful allogeneic transplant in patients who do not have matched allogeneic donor options. Strategies to decrease the HLA specific antibody titer may include antibody removal via TPE and IVIG, plasma cell depletion via rituximab and bortezomib and donor platelet infusion in cases of anti-HLA I antibodies. In certain cases, these strategies are not adequate to successfully deplete DSA.9
Daratumumab is an IgG1κ human monoclonal antibody directed against CD38, a cell surface glycoprotein highly expressed on plasma cells. It inhibits the growth of CD38 expressing cells by inducing apoptosis directly through Fc mediated cross linking, as well as by immune-mediated tumor cell lysis through complement dependent cytotoxicity, antibody dependent cell mediated cytotoxicity, and antibody dependent cellular phagocytosis. Daratumumab has been used in cases of ABO mismatch management and in solid organ transplant for prevention and treatment of organ rejection by depletion of DSA.8
Jordan et al treated two patients, one awaiting heart transplantation and the other with antibody mediated graft rejection, with daratumumab 16 mg/kg IV weekly for four doses. Daratumumab effectively reduced HLA antibodies to acceptable levels in one patient and improved graft rejection in the other.10 Curtis et al treated a 51-year-old patient with eight weekly doses of daratumumab resulting in a negative crossmatch result and subsequent successful heart transplant without evidence of graft rejection.11 Magda et al reported treating three critically ill patients awaiting lung transplantation with desensitization using daratumumab.12 No patient experienced severe primary graft dysfunction or hyperacute rejection. An ongoing study is recruiting patients with allosensitization or kidney transplant rejection to daratumumab dose escalation (phase 1) and full dose (phase 2) cohorts (NCT04204980).13
Although the mechanism of daratumumab would be expected to result in a more sustained effect on antibody titer, notably, our patient had a rebound increase in antibody titer after interruption of daratumumab resulting in a significant rise in MFI (higher than pre-treatment level). Therefore, periodic assessment of antibody level is essential even after DSA negativity has been documented.
In conclusion, daratumumab as part of a desensitization regimen appears to be safe and effective. Further experience to confirm the efficacy of this method and devise guidelines for treatment is required.