Mucormycosis (previously called zygomycosis) refer to infections caused by fungi of the subphylum Mucoromycotina, and include the genera Mucor, Rhizopus, Rhizomucor, Absidia, Apophysomyces, Cunninghamella, and Saksenaea.1 The most common types of infections include pulmonary, sinus, nasal, cutaneous, orbito-cerebral and disseminated, whereas the gastrointestinal tract is a rare site of involvement. Risk factors for infection include immunocompromising conditions such as a hematologic malignancy, solid organ transplant, hematopoietic stem cell transplant (HSCT), high dose corticosteroid therapy, diabetes mellitus, trauma and burns.2 A correlation with iron overload and deferoxamine therapy has also been suggested.3 An additional risk factor in HSCT patients is the presence of graft versus host disease (GVHD) which leads to added immunosuppressive therapy in these patients.
Mucormycosis has emerged as the third most common fungal infection in HSCT recipients after aspergillus and candida.4 Gastrointestinal (GI) mucormycosis is exceedingly rare and the diagnosis can be extremely challenging given the non-specific symptoms. We report a case of gastrointestinal mucormycosis with concomitant gastrointestinal graft versus host disease causing multiple intestinal and gastric perforations. A similar presentation has not been previously reported in the literature.
CaseA 39-year-old female with B cell acute lymphoblastic leukemia (ALL) underwent a double umbilical cord blood transplant (UCBT) while in first complete remission (CR1). The patient's blood type was B positive and she was CMV IgG positive. Both cord blood units were O positive and CMV negative. Conditioning regimen comprised of cyclophosphamide 50mg/kg, fludarabine 30mg/m2, thiotepa 5mg/kg and total body irradiation. GVHD prophylaxis comprised of tacrolimus and mycophenolate mofetil, and infectious prophylaxis included acyclovir, voriconazole and letermovir. Neutrophil engraftment occurred on day +17 and platelet engraftment on day +35. Post-transplant course was complicated by grade IV gastrointestinal GVHD treated with high dose corticosteroids, alpha-1 antitrypsin (AAT), budesonide and natalizumab on a study protocol. GVHD symptoms resolved by day +35.
On day +51, the patient was diagnosed with transplant associated microangiopathy with rising LDH, low haptoglobin, thrombocytopenia, elevated soluble C5b-9 and schistocytes on peripheral blood smear. This was thought to be due to the GVHD process and tacrolimus was continued while weekly eculizumab was started. On day +80, the patient was hospitalized with Klebsiella pneumoniae bacteremia and altered mentation not entirely attributable to the sepsis. Computed tomography (CT) scan of the head revealed an 18×18mm lucent focus in the left occipital lobe, interpreted as a possible area of infarction. Contrast-enhanced MRI of the brain showed scattered acute and subacute infarcts bilaterally along the cerebral and cerebellar hemispheres. The possibility of TMA-related strokes was entertained and tacrolimus was discontinued. Eculizumab was held in sight of the bacteremia and ruxolitinib 5mg twice daily was started.
The patient continued to have fever despite subsequent negative blood cultures that prompted further imaging. On day +115, a CT scan of the chest, abdomen and pelvis showed two low-density lesions along the inferior aspect of the left lateral lobe of the liver and MRI abdomen showed two subcapsular complex cystic liver lesions with faint rim enhancement in the left lateral lobe measuring 2.7 and 1.5cm, respectively. Of note, there was significant iron deposition in the liver and spleen. At this time the patient was on posaconazole which was held because of a rise in liver enzymes and caspofungin was started.
On day +118, the patient had a sudden episode of large volume melena with hemodynamic instability. CT angiogram (CTA) was negative for an actively bleeding source. The coagulation profile was normal. Flexible sigmoidoscopy showed limited mucosal visualization secondary to presence of blood clots in the recto-sigmoid, sigmoid and descending colon. The minimally visualized mucosa was normal. The episodes of melena continued requiring multiple PRBC and platelet transfusions. Meanwhile, the patient's mental status continued to wax and wane with intermittent refusal to care. A colonoscopy on day +123 showed a 2cm clean-based ulcer at the hepatic flexure. An upper endoscopy on day +130 showed a non-bleeding crated ulcer in the gastric body 2.5cm in largest dimension. Empiric treatment for CMV with ganciclovir was started.
On day +133, the patient developed new onset tachycardia, tachypnea and hypotension. CT of the abdomen and pelvis showed pneumoperitoneum, mesenteric gas and portomesenteric venous gas indicating intestinal perforation. An emergent exploratory laparotomy was done that showed areas of small bowel perforation and oozing of blood. Three segments of small bowel were resected. Pathology showed crypt cell apoptosis, crypt drop-out, multiple geographic ulcers, perforation, acute serositis and partial mucosal regeneration. Fungal colonization was noted in several ulcers and immunohistochemical staining for CMV was negative. Grocott's methenamine silver (GMS) stain showed ribbon-like septate fungal hyphae of irregular width suggestive of mucormycosis). The patient was started on isavuconazole.
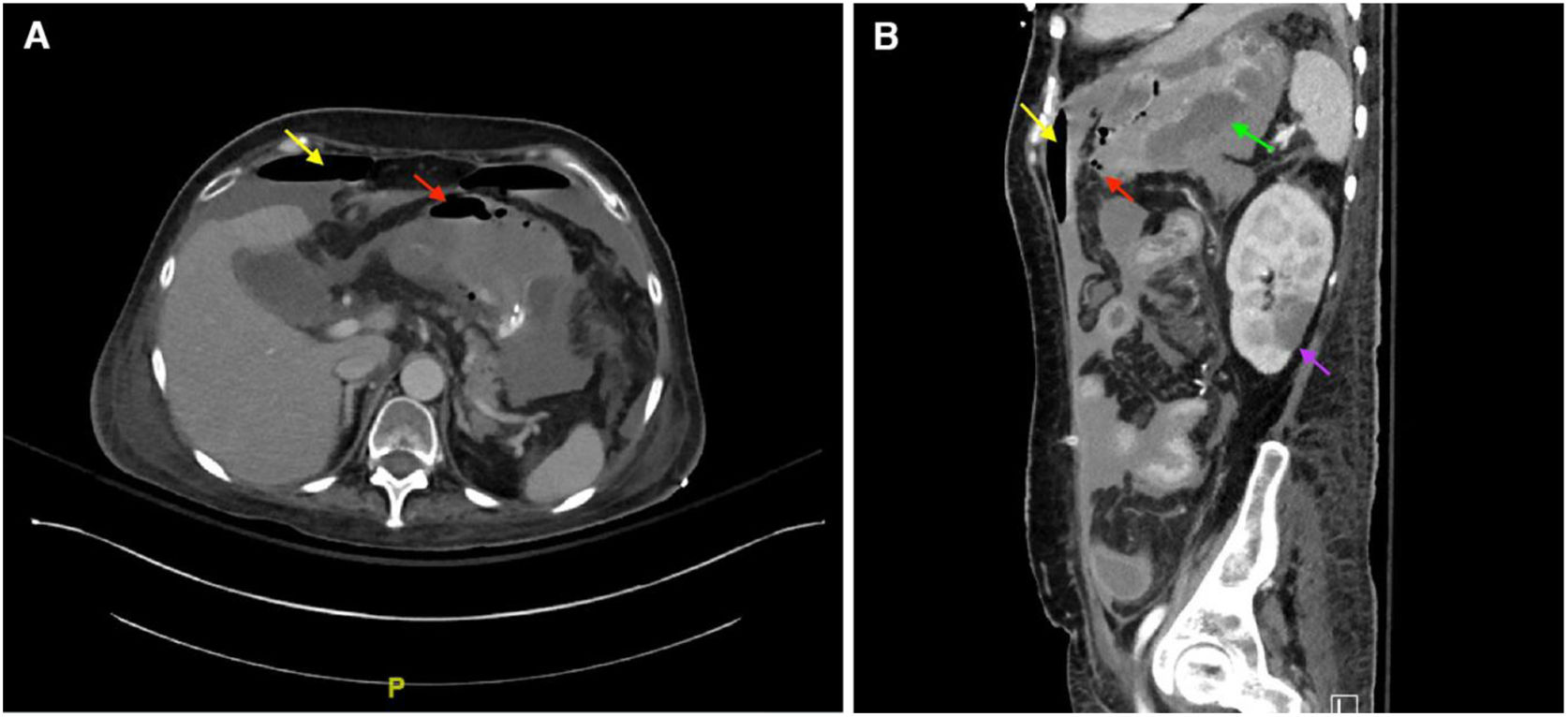
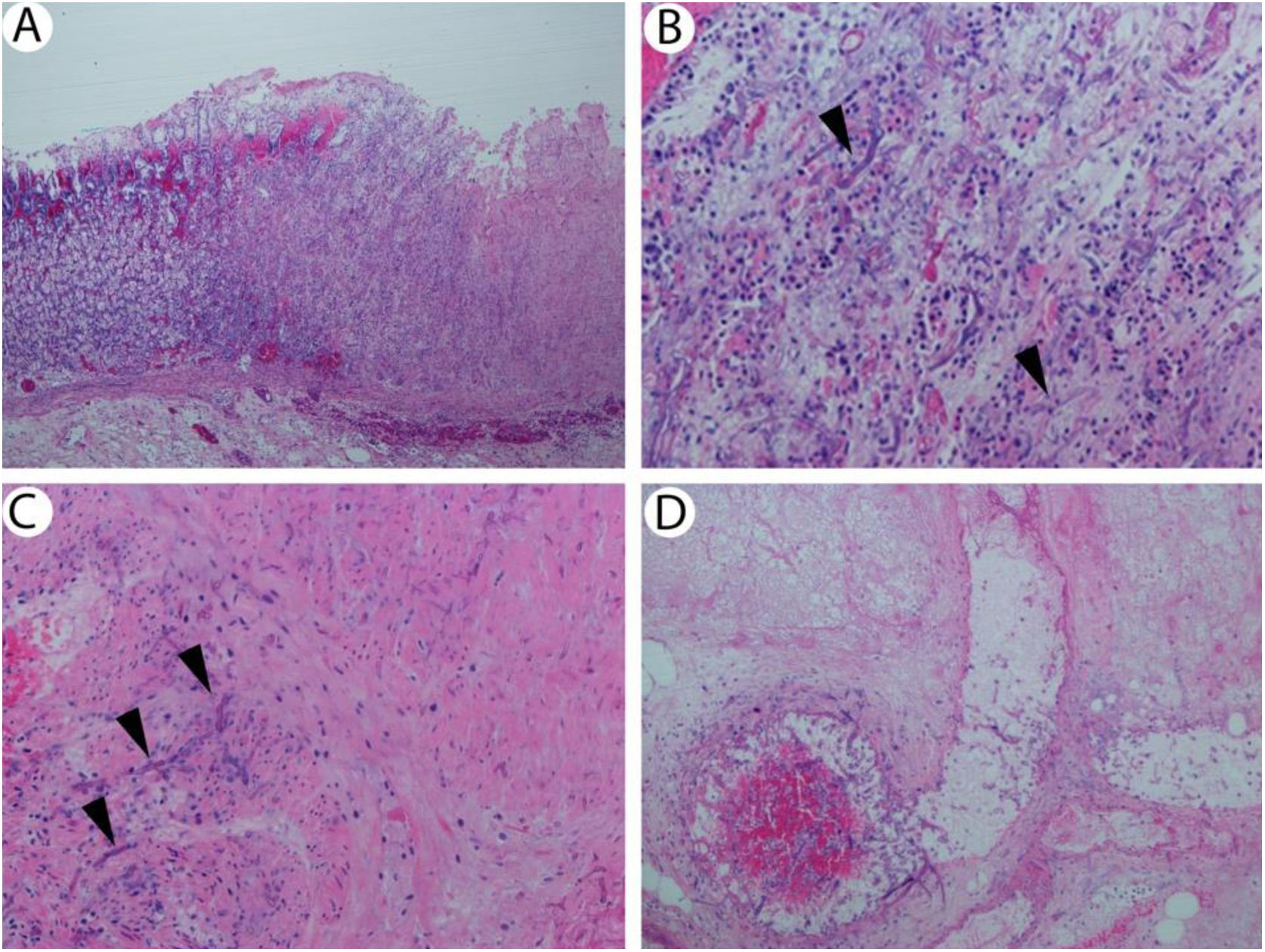
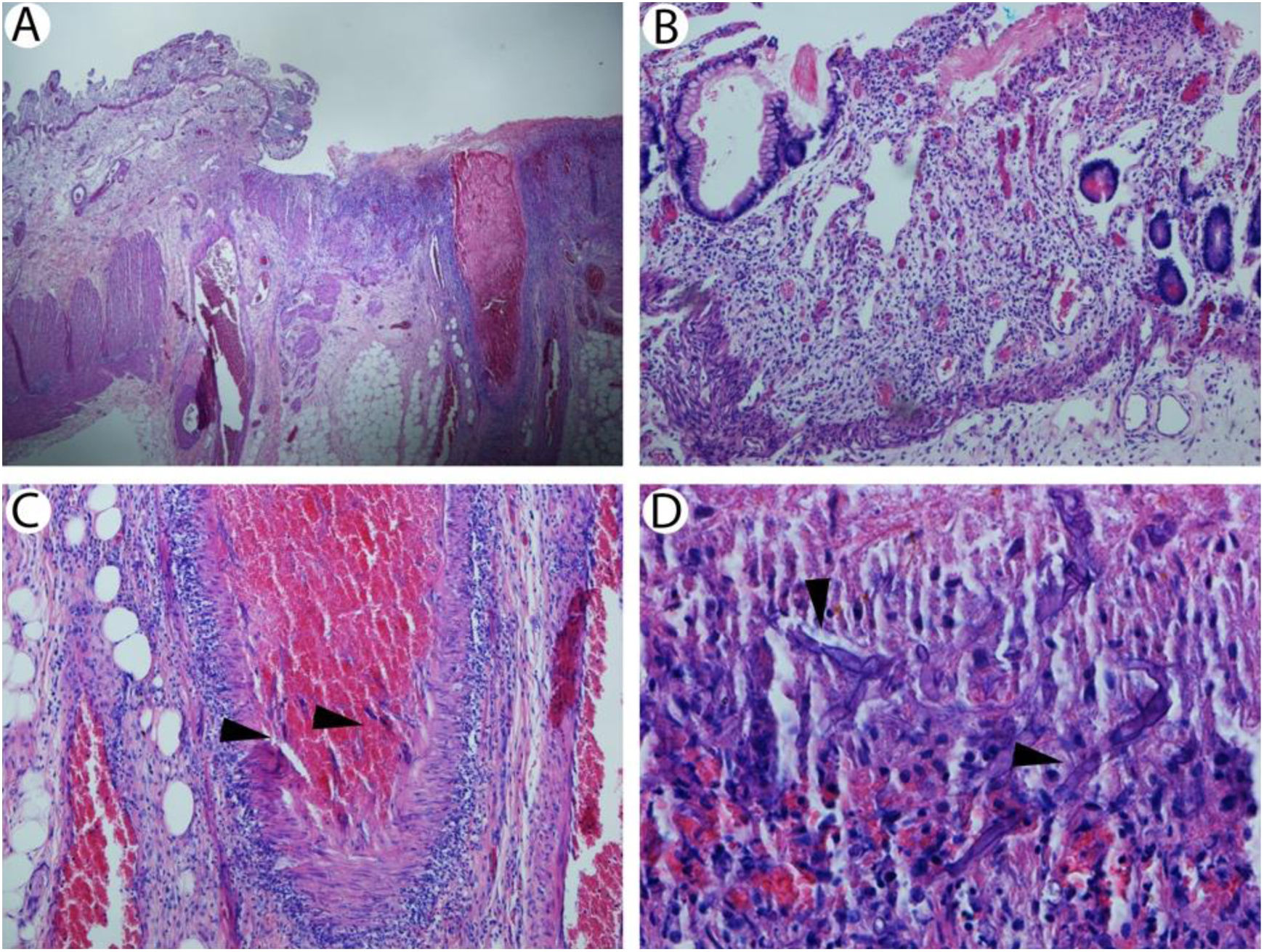
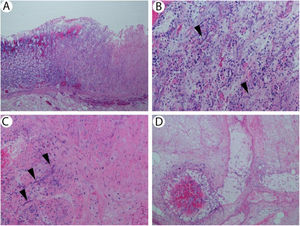
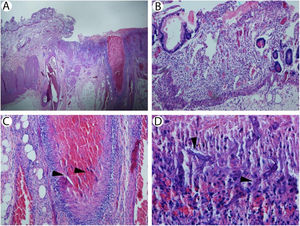
On post-operative day 4, the patient developed fever and a repeat CT scan of the abdomen showed new wall thickening of the greater curvature of the stomach and extraluminal gas at the lesser curvature concerning for perforation (Figure 1). After an extensive discussion regarding the risks and benefits of surgery, a decision for another exploratory laparotomy was made. A large perforation was seen in the lesser curvature of the stomach with circumferential necrosis, an area of necrosis involving the prior distal anastomosis site and multiple areas of ischemia in the small bowel. The patient underwent a distal gastrectomy, gastrostomy and duodenostomy. Pathology showed portion of the gastric fundus with transmural necrosis and associated fungal forms consistent with mucormycosis (Figure 2). Fungal forms were also seen to be clogging the blood vessels. A portion of the small intestine showed ulceration and necrosis centered on large blood vessels filled with fungi. The non-ulcerated small bowel mucosa showed extensive crypt dropout, but no significant apoptotic activity, consistent with prior episodes of severe graft versus host disease (Figure 3). Tissue culture grew Rhizopus species. Over the ensuing days the patient's condition further deteriorated and she died on day +144 post-transplant.
(A) Stomach with ulcer and fungi (hematoxylin & eosin, 40×). (B) Ulcerated and necrotic mucosa with ribbon-like fungal forms consistent with mucormycosis (arrowheads; hematoxylin & eosin, 200×). (C) Stomach wall showing fungi within muscularis propria (arrowheads; hematoxylin & eosin, 200×). (D) Necrotic vessel in stomach wall containing fungi.
(A) Small bowel with ulcer centered on large vessel (hematoxylin & eosin, 40×). (B) Non-ulcerated small bowel mucosa showing crypt dropout but no significant apoptotic activity consistent with prior episode of graft versus host disease (hematoxylin & eosin, 200×). (C) Artery in small bowel wall containing fungi consistent with mucormycosis (arrowheads; hematoxylin & eosin, 200×). (D) Necrotic small bowel mucosa with fungi consistent with mucormycosis (arrowheads; hematoxylin & eosin, 400×).
Gastrointestinal mucormycosis is a rare life-threatening infection primarily reported in immunocompromised hosts. Symptoms can be non-specific and a high index of suspicion influences the ability to make a prompt and timely diagnosis. The fungus invades tissues and blood vessels resulting in thrombosis and necrosis with resultant hemorrhage. Therefore, gastrointestinal mucormycosis can present as ischemic colitis and melena and the diagnosis must be suspected in patients with risk factors and no other obvious cause of gastrointestinal hemorrhage. Endoscopic examination may show ulceration and necrosis without an obvious site of bleeding.5 A definitive diagnosis requires positive tissue cultures and histopathologic demonstration of the fungi which appear as thick non-septate hyphae branching at right-angles.6 Risk factors in our patient included graft versus host disease, prolonged corticosteroid therapy, and ongoing bacterial infections. On pathologic examination, the necrotic ulcer in the small bowel was seen to be centered on the vessel filled with fungi. Small bowel wall denudation may have then led to episodes of large volume bleeding that obscured mucosal visualization on endoscopic examination.
In the largest reported single institution series of mucormycosis in 129 patients over a 10-year period (1990–1999), no cases of gastrointestinal mucormycosis were reliably diagnosed ante-mortem. All cutaneous, 91% of rhino-orbito-cerebral and 31% pulmonary cases were diagnosed ante-mortem.7 A French multi-center retrospective cohort study reported 29 cases of mucormycosis in 7097 HSCT recipients (prevalence 0.4%), 26 of whom were on steroids for GVHD treatment. Only one patient was reported to have gastrointestinal mucormycosis.8 Maertens et al. report a case series of five HSCT recipients of whom one had esophageal and gastric mucormycosis diagnosed on pathologic examination of an endoscopic biopsy specimen. The median time from HSCT to diagnosis was 343 days. Four patients had either acute or chronic GVHD requiring corticosteroid therapy. Of note, all patients had evidence of iron overload.3
When HSCT patients develop gastrointestinal symptoms, more common differentials such as GVHD and CMV colitis are first entertained. However, it is exceedingly rare for GVHD to be a cause for an abdominal surgical emergency such as intestinal perforation. In a review of 36 cases of gastrointestinal GVHD, Chirletti et al. reported seven patients with GI emergencies, three of whom required surgery. Four of the seven patients experienced severe GI bleeding and three had acute peritonitis, two of whom had an ileal perforation and cecal and left colon perforations, respectively. No patients had a gastric perforation.9 In a review of 63 patients with GI GVHD, only one patient required surgical intervention for multiple dilated loops of small bowel, however, there was no hemorrhage or perforation.10 Hence, with an atypical presentation in the presence of GVHD, an alternative differential and possibility of empiric treatment must be entertained while awaiting pathologic exam and tissue culture results.
Treatment of mucormycosis involves a combination of surgical debridement of involved tissue if deemed feasible and antifungal therapy. Intravenous amphotericin B (lipid formulation) is the drug of choice for initial therapy. Posaconazole or isavuconazole can be used as step-down therapy for patients who have responded to amphotericin B or can be used as salvage therapy for patients who do not respond or cannot tolerate amphotericin B.11 Initiation of a polyene antifungal therapy within six days of presentation is strongly associated with improved survival.12
ConclusionsDespite treatment of mucormycosis, the mortality in HSCT recipients remains unacceptably high.13 Therefore, a prompt diagnosis, elimination of predisposing factors such as reduction of immunosuppression, and timely initiation of antifungal therapy are critical. One must consider that in allogeneic HSCT recipients, mucormycosis often develops as a breakthrough infection, since these patients are already on antifungal prophylaxis as standard of care. Clinicians should thus be aware of the possibility of mucormycosis and its ability to cause angioinvasion and necrosis. An emergent multidisciplinary diagnostic approach should be undertaken to obtain tissue culture in the early stages of colonization before fulminant infection develops and initiate therapy to prevent fatal outcomes.
Conflicts of interestThe authors declare no conflicts of interest.