The disease produced by SARSCOV-2 had a significant impact around the world. According to the Peru's Health Ministry, on 22 August 2021, the percentage of positive cases was 13.04% and the fatality was 9.24%.1 Plasma convalescence was a treatment of choice, however, since the clinical trial RECOVERY did not demonstrate significant differences in 28-day mortality between the care group (24%) and the plasma convalescent group (24%).2
In response to the pandemic, we designed a phase II trial of convalescent plasma in patients with moderate COVID-19 (Register at the National Institute of Health of Peru #PER-03-020). Due to the | results of the RECOVERY trial, we decided to stop our study early after enrolling 10 convalescent donors, while no COVID-19 patients received the administration of convalescent plasma.
In this letter, we present the secondary aim of this trial that was to compare the biochemical, hematological, and serological values of the convalescent plasmas against COVID-19 with convalescent plasma donors obtained from volunteers’ apheresis.
The inclusion criteria for donors were an age between 18 and 60 years, a positive test for SARS-CoV-2 by RT-PCR of a nasopharyngeal sample, complete remission of symptoms at least 28 days before plasma donation or a negative test by RT-PCR of a nasopharyngeal sample and IgG anti-SARS-CoV-2 1: 200 determined by ELISA and. In addition, comply with the technical requirements for selecting human blood donors given by the Ministry of Health of Peru.3
Overall, 10 out of 35 candidates met the criteria issued by the Peruvian National Blood Program COVID-19 convalescent plasma.4 Seven donors presented antibodies against COVID-19 (ELISA Euroimmun dosing). The collection was from August 2020 to May 2021, obtaining 20 units of convalescent plasmas by collecting apheresis. In the blast freezer, the units were subjected to ultraviolet-based pathogen inactivation using riboflavin and rapid freeze plasma at -40°C. The evaluated analytes included factor VIII (by coagulometry with the Siemens kit), SARS-CoV2 antibodies IgG N antibodies of SARS-CoV2 (by Diasorin Chemiluminescent Immunoassay), total SARS-COV-2 (by the Roche electrochemiluminescence Immunoassay), SARS-CoV2 IGG antibodies of SARS-CoV2 (by enzyme-linked immunosorbent assay), C3 and C4 (by Roche Immunoturbidimetry) and total proteins (by Colorimetric Roche kit). R Studio was used to analyze the data collected.
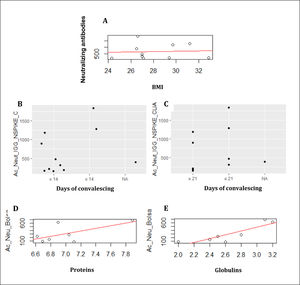
Although we had a very small sample size, we were able to find correlations between some clinical features of patients and SARS-CoV2 antibodies production. Body mass index (BMI) was directly related with the donor's neutralization antibodies (p= 0.8628; Figure 1A). Frasca, et al, described this correlation previously, in the study, the production of antibodies between thin and obese patients was compared, where it was observed that there is a tendency for thin patients to produce more antibodies.4
A. BMI were correlated with the production of neutralizing antibodies. B. The convalescence days were evaluated on 14 days. C. The convalescence assessed days on 21 days. The analysis of the donor´s bag. D. The correlation between proteins and neutralizing antibodies. E. The correlation between globulin and neutralizing antibodies.
Days of convalescent was related with production of antibodies, comparing before and after 14 days or 21 days (p=0000; Figure 1B and 1C). However, Seow et al reported that SARS-CoV-2 antibodies were detected in most infected people between 10-15 days after the presence of symptoms.5 In another study, patient donations were between 1 to 40 days from the onset of COVID-19 symptoms. To accept the donation, antibodies against SARS-CoV-2 (IgG) had to be detected between 1:100–1:3200.6
Regarding to biochemical markers, level of proteins was positively related to neutralizing antibodies of the units of convalescent plasma (p=0.12), Figure 1D. Antibody responses against the spike protein of SARS-CoV-2 have been shown to be detectable between 1 and 3 weeks from the onset of symptoms.7,8 On the other hand, the correlation between globulins and neutralizing antibodies was significant (p= 0.01; Figure 1E).
Although convalescent plasma had not efficacy in the treatment of COVID-19, we learned about the production of neutralizing anti-SARS-CoV-2 antibodies and clinical variables associated to it.
Statements and DeclarationsAuthors have no interests that are directly or indirectly related to the work submitted for publication.
We must make a special thanks to Romano Pissani and Daniela Medina, who were in charge of selecting the donors and the following processes.