Persistent hematuria is a chronic complication of sickle cell anemia (SCA) which can progress to chronic kidney disease. The practice of early detection of persistent hematuria in children with SCA in steady state is important for timely intervention.
ObjectiveTo determine the prevalence of persistent hematuria among children with sickle cell anemia in steady state and compare the result with that of a group of HbAA controls. The outcome will possibly strengthen the health policy on the need for regular screening for persistent hematuria in children with SCA.
MethodsChildren with sickle cell anemia, aged 2–18 years in steady state, were recruited consecutively from the sickle cell clinic at the University of Nigeria teaching Hospital Enugu. The controls were similarly recruited from the children’s outpatient clinic. To determine persistent hematuria, dipstick urinalysis and microscopy were performed for both subjects and controls at enrollment and repeated after four weeks.
ResultsOut of the 122 children with SCA studied, 5 (4.1%) had persistent hematuria. None (0%) of the 122 age- and gender-matched HbAA controls had persistent hematuria. This difference in prevalence of persistence between HbSS patients and HbAA controls was statistically significant (p = 0.02).
ConclusionPersistent hematuria still occurs significantly more among children with SCA, even among those in steady state. Routine urinalysis at follow-up visits in children with SCA is strongly recommended, as this will aid early detection and prompt management to prevent progression to chronic kidney disease.
Sickle cell anemia (SCA) is an autosomal recessive disorder that results from the inheritance of homozygous sickle hemoglobin (HbSS).1 It is one of the most common genetic diseases worldwide with the highest prevalence reported in sub-Saharan Africa, where 3 to 4% of the populations are affected.2 In Nigeria, the prevalence of SCA is about 20 per 1000 births, equaling 150,000 children born annually with the condition.3 Persons with SCA may present to the clinician in a steady state or in “crisis”. Renal complications in SCA include hematuria, hyperfiltration, renal hypertrophy, hyposthenuria, proximal and distal tubular abnormalities and chronic kidney disease (CKD). Although most cases of hematuria are self-limiting, it is important that they are investigated to identify persistent cases, as well as exclude more life-threatening underlying causes.4 Hematuria is a risk factor for CKD,5 and is a reliable pre-azotemic predictor of chronic kidney disease.1,6 This is due to the damage to the tubular epithelial cells by erythrocytes present in the urine,5 the engulfment, incorporation and destruction of erythrocytes in tubular epithelial cells are harmful to the tubular epithelium due to increased intracellular content of hemoglobin, a heme-containing protein.5 The urinary dipstick is the most common screening test for hematuria,7 with a sensitivity range of 91–100% and a specificity range of 65–99% in detecting 1–5 RBCs per high power field.8–11 Urinalysis is not routinely performed for children with SCA in steady state in most of the outpatient clinics. This may result in a delay in the diagnosis and treatment of children with persistent hematuria. The huge burden of CKD, especially in the developing world, where the attendant need for renal replacement therapy is difficult to initiate and sustain, underscores the need to emphasize prevention. Hence, early detection and treatment of risk factors/conditions of CKD is of paramount importance. Owing to the paucity of data on the burden of persistent hematuria in this environment, the study aimed to determine the prevalence and pattern of persistent hematuria in children with SCA in steady state.
Materials and methodsStudy site and durationThe study was undertaken at the sickle cell clinic and children’s outpatient clinic of the Department of Pediatrics, University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu state, Southeast Nigeria. The study was performed over an 8-month period from July 2017 to February 2018.
Study design and study populationThis was a descriptive cross-sectional study of children 2 to 18 years old with SCA (confirmed by hemoglobin electrophoresis), who were in steady state, defined as the absence of fever, skeletal or abdominal pain in the preceding four weeks, absence of blood transfusion in the preceding four months, as well as children who were not on any medication except routine drugs at presentation. To determine if persistent hematuria also occurred in children without sickle cell anemia, controls were recruited for the study. Respondents were recruited consecutively, and an interviewer-administered questionnaire was completed. The HbSS participants were recruited as they presented to the weekly sickle cell clinic, while HbAA controls were recruited in a similar manner, as they presented to the outpatient day clinic for follow-up after recovering from minor ailments.
Inclusion and exclusion criteriaThe minimum sample size, using prevalence from a previous study by Anigilaje et al.12 in Ilorin, Nigeria, was 122 cases and 122 controls. Children 2 to 18 years of age with SCA, as confirmed by hemoglobin electrophoresis, who were in steady state and whose parents gave consent, were recruited. Steady state was defined as the absence of fever, skeletal or abdominal pain in the preceding four weeks, absence of blood transfusion in the preceding four months, as well as children who were not on any medication except routine drugs at presentation. Children with symptoms and signs suggestive of urinary tract infection, those with history of exposure to radiopaque dye and drugs that decrease the reactivity of the dipstick, as well as those with a history of ingestion of nephrotoxic drugs, were excluded from the study. Also excluded were those with previous renal pathology, menstruating females, males with penile discharge, as well as children who had participated in competitive sports in the previous 24 h. Apparently healthy HbAA Controls of similar age and sex were similarly recruited, as they presented to the outpatient day clinic on follow-up from minor ailments, such as acute uncomplicated malaria, acute gastroenteritis, pneumonia and upper respiratory tract infection.
Study protocolFollowing recruitment, necessary socio-demographic data, participants past medical history, health status in the preceding four weeks and drug history were obtained and recorded in a semi-structured and interviewer-administered proforma. Physical examinations of the participants were performed to check for signs suggestive of a renal disease, such as peripheral edema, ballotable kidney or bladder enlargement. Anthropometry and blood pressure measurements were collected and interpreted, using appropriate age and gender centile charts. Spot clean catch and midstream urine were collected, and dipstick urinalysis was performed within one hour of urine collection. For participants with hematuria on dipstick, further urine microscopy was performed to confirm hematuria.8,13
For the controls, apparently healthy children on follow-up at the outpatient day clinic who met inclusion criteria, were recruited in a similar manner as the cases. Hemoglobin genotyping was performed at recruitment, while urine examination was accomplished in a similar way as the cases. The result of the Hb genotype was communicated to the caregivers over the phone and those who were HbAA had repeat tests as the cases, while those with HbAS were subsequently excluded from the study.
In keeping with the study protocol, repeat tests were performed on all participants at four-week intervals. Participants with negative results at both instances were regarded as negative, while those with two positive tests, at the first and repeat tests, were regarded as being positive for hematuria and had no third clinical visit for repeat testing. Participants with a negative and positive test at initial and repeat testing, or vice-versa, were invited for a third clinical visit for a ‘tie breaker’ testing, to confirm or refute persistent hematuria. Social classification (occupation and educational background of both parents or guardian) was performed according to Oyedeji’s classification.14
Statistical analysisStatistical analysis was accomplished with the IBM-SPSS version 20.0 (Chicago II). Results were displayed in frequencies and tables, as appropriate. Normally distributed data were analyzed by parametric statistics, while non-normally distributed data were analyzed by non-parametric statistics. Group comparisons were made using the chi square test (categorical variables such as gender) and t-tests (for continuous variables, such as mean age, height, weight). Prevalence of persistent hematuria in children with HbSS and HbAA were compared using Fisher’s exact test. Renal sizes (length) of the children with persistent hematuria were compared with local and internationally available percentile charts. The p-values less than 0.05 were regarded as significant.
Ethical aspectsPrior to commencement of the study, ethical approval was obtained from the Health Research and Ethics Committee of the University of Nigeria Teaching Hospital, Ituku-Ozalla. Written informed consent were obtained from caregivers of participants prior to enrollment. Assent was also obtained from participants seven years old and over. Those with persistent hematuria were referred to the pediatric nephrology clinic.
ResultsThe mean age for sex and age-matched study participants were 10.1 ± 4.7 years and 10.1 ± 4.6 years, respectively, for children with SCA and controls. They also had similar gender distribution in both HbSS and HbAA (p = 0.89). The mean weight and height for study participants with HbSS were 31.5 ± 12.9 kg and 136.2 ± 23.4 cm, while those for participants with HbAA, who served as controls, were 32.4 ± 15.3 kg and 135.7 ± 24.6 cm, respectively (p < 0.05).
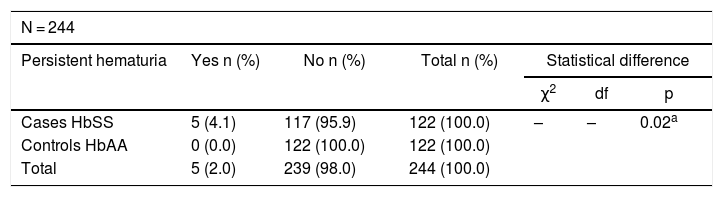
The prevalence rate of hematuria at first testing was 5.7% (7/122) among children with sickle cell anemia. On follow-up testing four weeks later, the prevalence rate of persistent hematuria was 4.1% (5/122). Two out of these seven cases had no hematuria on dipstick and microscopy on repeat testing. Comparatively, none (0%) of the 122 controls had hematuria at enrollment or follow-up, (p = 0.02), Table 1.
Prevalence of persistent hematuria among the participants.
| N = 244 | ||||||
|---|---|---|---|---|---|---|
| Persistent hematuria | Yes n (%) | No n (%) | Total n (%) | Statistical difference | ||
| χ2 | df | p | ||||
| Cases HbSS | 5 (4.1) | 117 (95.9) | 122 (100.0) | – | – | 0.02a |
| Controls HbAA | 0 (0.0) | 122 (100.0) | 122 (100.0) | |||
| Total | 5 (2.0) | 239 (98.0) | 244 (100.0) | |||
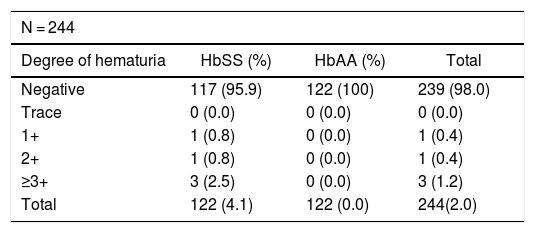
The details of the result of dipstick urinalysis for all study participants is shown in Table 2.
Degree of persistent hematuria among children with SCA (HbSS) and normal controls (HbAA) in dipstick urinalysis.
| N = 244 | |||
|---|---|---|---|
| Degree of hematuria | HbSS (%) | HbAA (%) | Total |
| Negative | 117 (95.9) | 122 (100) | 239 (98.0) |
| Trace | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1+ | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| 2+ | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| ≥3+ | 3 (2.5) | 0 (0.0) | 3 (1.2) |
| Total | 122 (4.1) | 122 (0.0) | 244(2.0) |
1+ = ≥5–10 RBCs/μL, 2+ = ≥25 RBCs/μL, 3+ = ≥50 RBCs/μL, 4+ = ≥250 RBCs/μL.
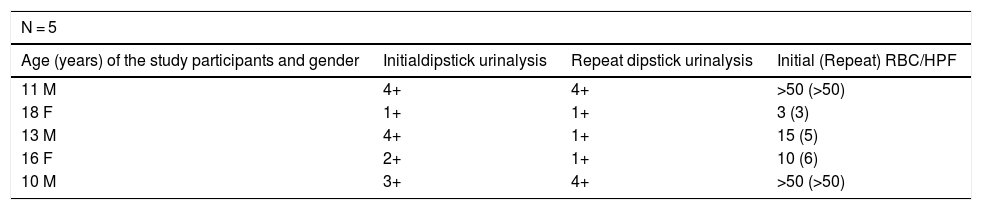
Microscopy was used to further confirm the presence of hematuria and the RBC count in children with positive urinalysis dipstick as shown in Table 3.
Results of urine dipstick and follow-up urine sediment microscopy for the five children with hematuria at initial and repeat test.
| N = 5 | |||
|---|---|---|---|
| Age (years) of the study participants and gender | Initialdipstick urinalysis | Repeat dipstick urinalysis | Initial (Repeat) RBC/HPF |
| 11 M | 4+ | 4+ | >50 (>50) |
| 18 F | 1+ | 1+ | 3 (3) |
| 13 M | 4+ | 1+ | 15 (5) |
| 16 F | 2+ | 1+ | 10 (6) |
| 10 M | 3+ | 4+ | >50 (>50) |
+ = degree of hematuria on dipstick urinalysis.
1+ = ≥5–10 RBCs/μL, 2+ = ≥25 RBCs/μL, 3+ = ≥50 RBCs/μL, 4+ = ≥250/RBCs/μL.
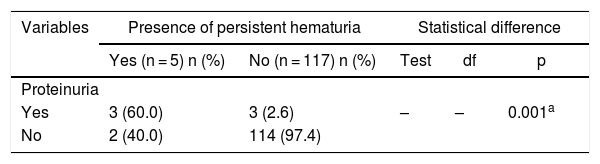
The association between the presence of hematuria and proteinuria was further analyzed and is shown in Table 4.
Relationship between persistent proteinuria and persistent hematuria in children with SCA.
| Variables | Presence of persistent hematuria | Statistical difference | |||
|---|---|---|---|---|---|
| Yes (n = 5) n (%) | No (n = 117) n (%) | Test | df | p | |
| Proteinuria | |||||
| Yes | 3 (60.0) | 3 (2.6) | – | – | 0.001a |
| No | 2 (40.0) | 114 (97.4) | |||
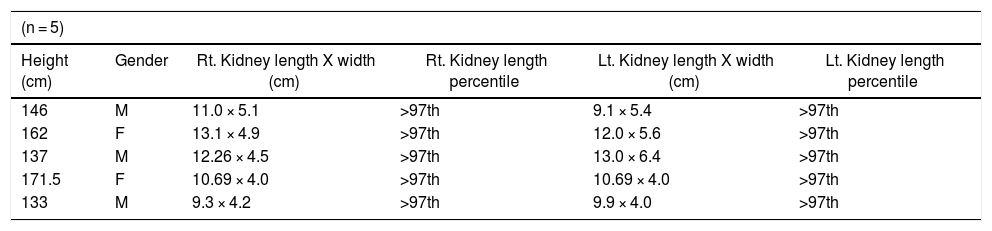
In those with persistent hematuria, we further examined their kidneys using ultrasound. Ultrasonography showed that four out of the five (80%) with persistent hematuria had normal echogenicity, while one of the five (20%) had hyperechogenicity. When compared to the local reference, all children with persistent hematuria had renal hypertrophy with left and right renal lengths greater than the 97th percentile, Table 5.
The renal sizes among Sickle Cell Anemia children with persistent hematuria compared with local standard.15
| (n = 5) | |||||
|---|---|---|---|---|---|
| Height (cm) | Gender | Rt. Kidney length X width (cm) | Rt. Kidney length percentile | Lt. Kidney length X width (cm) | Lt. Kidney length percentile |
| 146 | M | 11.0 × 5.1 | >97th | 9.1 × 5.4 | >97th |
| 162 | F | 13.1 × 4.9 | >97th | 12.0 × 5.6 | >97th |
| 137 | M | 12.26 × 4.5 | >97th | 13.0 × 6.4 | >97th |
| 171.5 | F | 10.69 × 4.0 | >97th | 10.69 × 4.0 | >97th |
| 133 | M | 9.3 × 4.2 | >97th | 9.9 × 4.0 | >97th |
cm = centimeters.
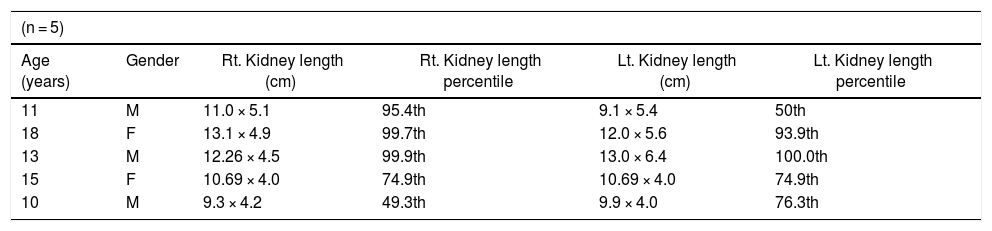
However, when compared with the international reference, 60% of these children had renal hypertrophy with their right renal length greater than the 95th percentile, while 20% had left renal length greater than 95th percentile, Table 6.
The renal sizes among children with persistent hematuria in SCA compared with international reference standard.16
| (n = 5) | |||||
|---|---|---|---|---|---|
| Age (years) | Gender | Rt. Kidney length (cm) | Rt. Kidney length percentile | Lt. Kidney length (cm) | Lt. Kidney length percentile |
| 11 | M | 11.0 × 5.1 | 95.4th | 9.1 × 5.4 | 50th |
| 18 | F | 13.1 × 4.9 | 99.7th | 12.0 × 5.6 | 93.9th |
| 13 | M | 12.26 × 4.5 | 99.9th | 13.0 × 6.4 | 100.0th |
| 15 | F | 10.69 × 4.0 | 74.9th | 10.69 × 4.0 | 74.9th |
| 10 | M | 9.3 × 4.2 | 49.3th | 9.9 × 4.0 | 76.3th |
The prevalence of persistent hematuria in SCA in this study was 4.1%, which was significantly different from prevalence of 0% in the HbAA controls. This difference is most likely due to the absence of capillary congestion caused by the persistence of sickled cells in the renal medulla of children with HbAA. This finding thus further strengthens the need to keep assessing for hematuria in SCA patients, even in those in “steady state”.
Though significant, this hematuria prevalence in our cohort of children with HbSS is low compared to other reports from the region; Anigilaje et al.12 in Ilorin, Ugwu et al.17 in Port Harcourt, Saad et al.18 in Zaria and Sayed19 in Sudan, who reported 13.3%, 11.1%, 8.8% and 23.2% of persistent hematuria, respectively, despite having studied children within a similar age bracket. Methodological differences, such as the inclusion of SCA patients in crisis, were performed by Saad et al.18 in Zaria and Sayed et al.19 in Sudan, while our study population was restricted to those in steady state, and this may account for the higher prevalence in the latter studies.18,19 This is plausible, as a significantly higher hematuria prevalence is reported among children with SCA in crisis, when compared to their counterparts in steady state.18 Repeated sickling and vascular obstruction in the renal medulla, with consequent extravasation of red blood cells in patients in crisis, may result in hematuria.20–22 Furthermore, Saad et al.18, applied the suprapubic method of urine collection in infants, moribund patients and children who were not toilet trained. Transient and microscopic hematuria has been reported as a common complication of suprapubic urine aspiration.23,24 This may have also have accounted for more cases of hematuria in that study.18 This was further shown by the higher prevalence among the age group where suprapubic collection was applied.18 In terms of methodology, the prevalence in Zaria by Saad et al.18 and in Sudan by Sayed19 were reported from a single urine sample examination and cases of transient hematuria and not “strictly” persistent hematuria may have been included. Among the cohort of SCA children studied by Sayed et al.19 in Sudan, 50% were reported to have severe disease, with very frequent vaso-occlusive events. This may account for the very high prevalence rates of hematuria reported.
Compared to the higher reported prevalence in other studies in Nigeria more than a decade ago, advances in SCA management, with the advent of evidence-based pharmacological and psychosocial treatments, have occasioned a better managed SCA child, who is more likely to have fewer vaso-occlusive crises (VOCs) and less hematuria. Comprehensive Health Care management of SCD, aimed at the prevention and early recognition of the complications of the disease, have also been increasingly employed in the country within the last decade, leading to an appreciable decrease in the morbidity and mortality due to SCA.25 This may inadvertently affect the occurrence of persistent hematuria. The prevalence reported in this index study is in agreement with that found in Ghana by Osei-Yeboah and Rodrigues,25 with the prevalence of 3.3% in children with HbSS and 0% in HbAA controls. This may be because both countries are in West Africa and predominantly have the intermediate type SCA severity, (the BENIN haplotype), hence the similar prevalence. Osei-Yeboah and Rodrigues26 also studied only SCA children in steady state, thus using a sample similar to that used in this index study.
In this current study, there was a significant positive association between hematuria and proteinuria. The pathophysiology of hematuria and proteinuria in SCA are sequential in occurrence and can explain the co-morbidity in these children. The vaso-occlusive process causes decreased blood flow in the renal medulla and results in medullary hypoxia, ischemia, necrosis and hematuria. The consequent compensatory hypoxia-induced prostaglandin secretion causes renal vasodilation which results in hyperfiltration, glomerulosclerosis and proteinuria.18 Hence, this comorbidity in these children may suggest a progressive or significant degree of sickle cell nephropathy. Ultrasonography, however, showed that only one of the five had hyperechogenicity and may have been the subject who already had a progressive pathology, despite being in a stable steady state. Suggested etiology of increased renal echogenicity include renal papillary necrosis, elevated iron deposits within tubular epithelial cells, focal scarring and interstitial fibrosis in the vasa recta system, glomerular hypertrophy and renal sclerosis.27 The other children with hematuria who had normal echogenicity, may mean that these structural renal changes associated with hyperechogenicity were yet to develop and may require follow-up studies. These findings thus suggest the need to emphasize use of routine urinalysis for earlier detection of renal pathologies in steady state SCA before more severe and permanent pathological changes begin to occur.
LimitationsWe excluded children in crisis, which may have also added valuable comparative information. Also, further evaluation, such as renal ultrasound on all study subjects, may have given better insight into the etiology of persistent hematuria. However, this was beyond the scope of the current study objective.
ConclusionThe percentage of persistent hematuria in the studied sample was significantly higher among children with sickle cell anemia in steady state, compared to the HbAA controls. The known endpoint consequences of persistent hematuria and the finding of persistent hematuria in stable SCA patients buttresses the need for regular urine screening in all children with SCA. Routine urinalysis at follow-up visits will aid early detection and prompt management to prevent progression to chronic kidney disease.
Conflicts of interestThe authors declare no conflicts of interest.
Author contributionAU-conceived the study and carried out the design of the study, acquisition of data, interpretation of data and manuscript writing.
AA-drafting the manuscript, manuscript writing and revising it critically for important intellectual content.
EO-the design of the study, interpretation of data and manuscript writing.
OHU-drafting the manuscript and revising it critically for important intellectual content.
EI-manuscript writing and revising it critically for important intellectual content.
All authors read and approved the final manuscript.