Immunoglobulin represents the main therapy for patients with inborn errors of immunity (IEI) and it is a safe procedure, but adverse events (AEs) can occur with variable frequencies.
ObjectiveTo evaluate the frequency of immediate AEs to intravenous immunoglobulin (IVIG) regular therapy in a pediatric cohort with IEI after a pre-IVIG infusion protocol.
MethodsThis was a longitudinal study from 2011 to 2019 at a tertiary pediatric hospital in Brazil.
ResultsA total of 1736 infusions were studied in 70 patients with IEI, 46 (65.7%) of whom were males and whose median age was 5.8 years old (range: 6 mo – 18 yo). Seven different brands of IVIG were used with the median loading dose of 0.57g/kg (range: 0.23 - 0.88g/Kg). According to the protocol, pre-medication and step-up infusion rate, were performed in 1305 (75.2%) infusions. Immediate AEs were noted in 10 children (14.3%) and in 22 (1.2%) infusions. Skin reactions (rash or urticaria) were the most common AE with 14 episodes (0.8% of all infusions). Almost all AEs were mild (19/86.4%), with no severe ones being observed. The majority of the AEs (81.8%) was identified at a 0.04ml/kg/min infusion rate. Gender, age at first infusion, presence of infection on the infusion day and change of the IVIG brand were evaluated and none of them were associated with AEs.
ConclusionThe low frequency of immediate AEs in children with IEI highlights the safety and tolerability of intravenous immunoglobulin replacement with the procedures established at our center.
Intravenous immunoglobulin (IVIG) is a well-known therapeutic approach for patients with inborn errors of immunity (IEI) with impaired antibody production or function, as well as for autoimmune and autoinflammatory conditions, such as the Kawasaky disease and autoimmune thrombocytopenia.1-3 Human Immunoglobulin (Ig) preparations are derived from a plasma pool and contain usually more than 95% protective titers of IgG against a large number of pathogens with traces of IgM and IgA, depending on the purification and fractionation process.2,4-6
The IVIG replacement therapy reduces the frequency and severity of infections and reduces the number of hospital admissions, as well as improves the quality of life in patients with IEI.7-11 It is usually safe and well tolerated, but adverse events (AEs) can occur and the frequency is highly variable, usually ranging from 1 to 40%, in patients with IEI.12-16 Most cases of AE are related to the infusion rates and are immediate (in the first 6 hours of the infusion) with mild symptoms, although severe AEs can happen in around 10% of patients, depending on the study population.12,14,18-20 The mechanism has been associated with the IVIG preparation, such as the antigenicity of the IgG itself, large-molecular weight IgG aggregates, presence of an antibody to a circulating pathogen or self-antigens, complement activation, or related to the patient characteristics, such as age, active infection and baseline disease.3,12,17,18
In 2011, a pre-infusion protocol was established at a tertiary pediatric hospital to evaluate AEs to IVIG in patients with IEI under regular therapy and data from an eight-year follow-up of 70 children are described in the present report.
MethodsPatients and infusion protocolThe inclusion criteria were: (i) age from 1 month to 18 years of age during the therapy; (ii) diagnosis of IEI based on the International Societies for Primary Immunodeficiencies,21-23 and; (iii) regular Ig replacement therapy with at least one infusion, according to the Brazilian guidelines for patients with IEI.4 The patients under IVIG therapy with secondary immunodeficiency were not included. The study was conducted at a pediatric tertiary hospital in Brazil and was approved by the Brazilian Ethical Committee Review Board.
The protocol for IVIG infusion and evaluation of infusion-related AE was established in 2011 and includes: 1) No pre-medication, in cases of more than three infusions with non-switched brand/batch: patients receive 0.9% saline solution 30 minutes pre- and post-IVIG (infusion rate of 5ml/kg/h) and symptomatic if necessary, and; 2) Pre-medication, in cases of first three infusions, switching IVIG brand/batch or presence of active infection: patients receive hydrocortisone (5mg/kg, intravenous injection) and antihistamines (oral dexchlorpheniramine maleate or parenteral diphenhydramine hydrochloride) 30 minutes pre-infusion, as well as saline solution pre- and post-infusion. In both conditions, interruption of infusion and antiemetic/antipyretic were used in case of AE. Infusion data are recorded by nurses and include brand and batch preparations, clinical condition of the patient (such as vital signs), pre-treatment medication, infusion rate, adverse reaction signs and symptoms, medications used to treat reactions and information obtained during follow-up evaluations.
Data were collected until October 2019, including: gender, IEI diagnosis, age, active infection, step-up infusion rate, pre-treatment medication, IVIG brand, rate of infusion, number of batches, symptoms of AE, interruption of infusion (temporary or complete) and medication to treat AE. Patients receive IVIG therapy under supervision of the same team of clinical immunologists and trained nurses.
Adverse events (AE)Immediate adverse reactions were defined as: started within six hours from the initiation of the infusion, or any time before the termination of the infusion, for those whose infusion lasted more than six hours and were diagnosed by a physician.12,20 The AEs were classified as the following:
- i
mild reactions: headache, flushing, muscle aches, shivering, feeling sick, itching, urticaria, anxiety, lightheadedness, dizziness or irritability. These subsided when the infusion rate was decreased;
- ii
moderate reactions: mild reactions becoming worse, or other symptoms, such as chest pain or wheezing, necessitating the infusion to be discontinued, and;
- iii
severe reactions: moderate reactions persisting or becoming worse, or other symptoms, such as tightness of the throat, severe headache and shaking, severe breathlessness or wheezing, severe dizziness or fainting, sensation of pressure in the chest or collapse.13
Seven brands of IVIG preparations were infused during the study period. Endobulin Kiovig® (Baxter AG, Vienna, Austria), Octagam® (Octapharma Pharmazeutika, Vienna, Austria), Flebogama® (Grifols, SA, Barcelona, Spain), Immunoglobulin® (GCC; Swon-City, Korea), Tegeline® (LFB-biomedicaments, Les Ulis, France), Sandoglobulina® (CSL-Behring AG, Bern, Switzerland) and Privigen® (CSL-Behring AG, Bern, Switzerland). All preparations infused were provided by the Brazilian government.
Statistical analysisData are described as frequency, median, mean, range and interquartile range (IQR). The analysis was performed with the IBM SPSS (Statistical Package for the Social Sciences) ® 23, 2015. The Kolmogorov-Smirnov´s test was used as the normality test and the X2 test was used for quantitative and related samples. To study the possible influence of multiple infusions in a single patient, logistic regressions (multivariate analysis) were adjusted to consider the effect of the patient identity as a random effect. The comparison between the time reaction and severity was made using the Mann–Whitney U test. Statistical significance was considered when the p-value was less than 0.5.
ResultsPatients and infusion characteristicsIn total, 70 patients with IEI in IVIG therapy, median age 5.8 years old (range: 6 months - 18 years old) were included, with a predominance of males, 46 (65.7%). No difference was noted when patients were stratified by age group: infants and toddlers (24.3%), preschool (24.3%), school (25.7%) and adolescents (25.7%). According to the IEI group,23 patients were classified as: predominantly antibody deficiencies (PAD) (n = 62, 88.6%), combined immunodeficiencies with associated or syndromic features (n = 7, 10.0%) and Immunodeficiencies affecting cellular and humoral immunity (n = 1, 1.4%).
During the study period, a total of 1736 infusions were administered (median: 18.5/patient, range: 1 - 58/patient), distributed according to the IEI group as follows: “PAD” 1509 (86.9%), “combined immunodeficiencies with associated or syndromic features”, 208 (12%), and “Immunodeficiencies affecting cellular and humoral immunity”, 19 (1.1%).
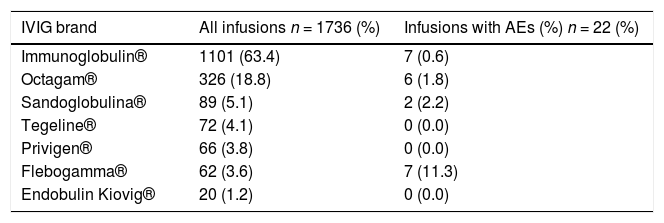
The mean loading dose of IVIG was 0.57g/kg (range: 0.23 - 0.88g/Kg). The pre-medication and step-up infusion rate were observed in two-thirds of the infusions (n = 1305, 75.2%). Each patient received different brands of IVIG according to the availability of the Brazilian government supply. The preparations used were as follows: Immunoglobulin® (1101, 63.4%), Octagam ® (326 /18.8%), Sandoglobulina® (89, 5.1%), Tegeline® (72, 4.1%), Privigen® (66, 3.8%), Flebogamma® (62, 3.6%), Endobulin Kiovig® (20, 1.2%).
Adverse events to immunoglobulinIn total, immediate AEs were observed in 22 (1.2%) infusions in 10 children (14.3%). The median age of patients who presented AEs was three years old (range: 10 months - 17 years old) and five (50.0%) were males. The predominant diagnosis was specific antibody deficiency (SAD) (5/50%), followed by Transient Hypogammaglobulinemia of Infancy (THI) and unclassified hypogammaglobulinemia (UH), two cases each and one patient with ataxia-telangiectasia (AT).
Among the patients who had AEs, most of them had associated comorbidities previous to the IVIG therapy: autoimmune cytopenia (n = 2), cardiac disease (n = 2), asthma (n = 1) and glucose-6-phosphate dehydrogenase (G6PD) deficiency (n = 1). The patient with AT developed an ovarian cancer during the follow-up. Her past history of drug adverse reaction or allergic disease were negative.
The distribution of brands of IVIG and AEs is shown in Table 1. The brand Immunoglobulin® was used in more than half of the infusions (63.4%). No AEs were observed with the brands Tegeline®, Privigen® and Endobulin Kiovig®, used in 158 (9.1%) infusions.
Distribution of infusions and adverse events according to IVIG brands in 70 patients with inborn errors of immunity (2011 - 2019).
IVIG: intravenous immunoglobulin, AEs: adverse events.
Skin reactions (rash or urticaria) were the most common AEs, with 14 episodes (63.6%); interestingly, 12 out 14 of these episodes occurred in two patients. Almost all AEs were mild (19, 86.4%), three moderate (13.6%) and no severe were observed (Figure 1). The mean loading dose in the 22 infusions with AEs was 0.56g/Kg (range: 0.23 - 0.78g/kg).
It is worth noting that all moderate immediate AEs were observed in the same patient, a 2-year-old boy, with UH (baseline IgG serum level < 200 mg/dL), with recurrent respiratory and gastrointestinal infections in association with a positive history of IEI (his older brother had died due to sepsis at 1 yo). This patient also had a G6PD deficiency, but no clinical feature of allergic or other chronic/autoimmune disease. He received Flebogamma® in all three infusions, however, as no other patient had a moderate AE with this brand, there is not enough data to establish a correlation. In this particular case, after recurrent episodes of skin rash/chills, 6 out of 8 infusions, the intravenous route was changed to subcutaneous with the resolution of reactions.
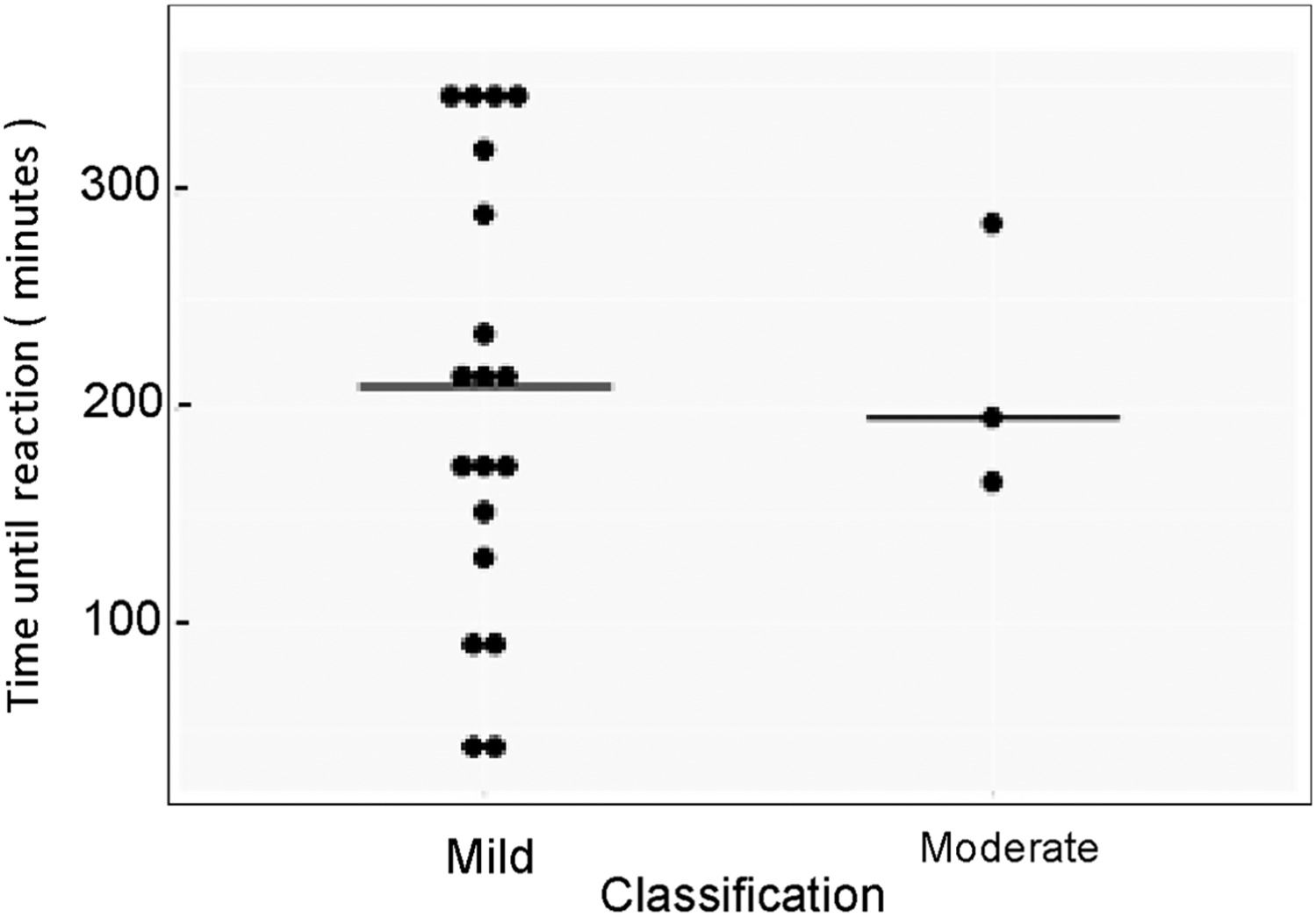
No significant association was observed in the distribution of latency by severity of the AE, p > 0.05 (Figure 2). However, most AEs occurred at 0.04ml/kg/min rate, n = 18 (81.8%) and three (13.7%) at 0.02ml/Kg/min. In 10 reactions, the infusion was completely interrupted. It is worth noting that in 20 infusions with AE, the patients had already received pre-medication (Supplementary Table).
The age, changing brands, active infection and first three infusions were studied as potential independent risk factors for AEs in our patients. Logistic regression analysis was performed and no statistical association was found (Table 2).
Potential risk factor associated for adverse effects related to IVIG at replacement dose.
IVIG: Intravenous immunoglobulin, CI: confidence interval of 95%.
Complete (36.4%) or temporary (66.6%) interruption of IVIG infusion and administration of symptomatic medications reverted all reactions. In cases of temporary interruption, the infusion restarted with the maximum loading dose of 0.03ml/kg/min.
DiscussionImmunoglobulin represents the main therapy for patients with impaired antibody production or function, with great impact on the quality of life for many patients.4,5,8,10 It also has benefits in several diseases that manifest with hypogammaglobulinemia, including graft-versus-host disease, multiple myeloma and chronic leukemia.3 or in patients with immune dysregulation.1 It is considered a safe procedure, but adverse events are not uncommon and these reactions can be local (at the infusion site) or systemic and immediate (within 6 hours of the infusion) or delayed (6 hours to one week after the infusion).3,12,13,19 The main strategies described to prevent adverse reactions triggered by immunoglobulin infusion are: controlling triggering factors, hydrating the patient before infusion, infusing the product at room temperature, monitoring vital signs during infusion, infusing at a slower infusion rate in the first administrations and keeping the patient under observation for one hour after the end of the infusion.3,12,24 Even with the knowledge of triggering factors, the percentages of AEs related to the IVIG can be up to 40% of infusions at replacement doses.14,15 The present study describes the frequency of immediate AEs in patients with antibody deficiency under regular IGIV replacement therapy, using a pre-infusion protocol and, as far as we know, it is the first report on a pediatric cohort with IEI.
A total of 1736 infusions in 70 patients were evaluated and the incidence of AEs was very low, being 1.2 % (n = 22) of infusions. This finding was lower than most studies enrolling a pediatric population with IEI.13,15,18-20 This discrepancy can be related to the protocol described here, or in other words, the use of pre-medications in the first three infusions or in case of switching the IVIG brand. We are quite sure that it had a great impact on our results, as this medical strategy was adopted in 75.2% of all infusions, it also reflects the high frequency in brand switching and, without pre-infusion strategies, the proportion of AEs perhaps could be higher.25 The IVIG preparations have several differences in formulation and are not equally tolerated;4,24,26 thus, it has been recommended that changing in brands should be done with caution4,25 and indicated by the physician,4 while unfortunately, as described herein, our patients rarely receive the same IVIG in two infusions in a row.
Most of the reactions were mild (86.4%) as expected, three, moderated (cyanosis and dyspnea) in a single patient and no severe reactions. Mild symptoms are predominant in patients with IEI, especially chills, fever and headache and usually have resolution with temporary infusion and symptomatic medication.12-14,18,27 Severe AEs are more frequent in patients with autoimmunity and neurological disorders and hematological diseases treated with higher IVIG dose.3,12,24 However, it also can happen in antibody-deficient patients; Bichuetti-Silva and colleagues observed a relevant rate of 7.9% infusions with severe reactions18 and Esmaeilzadeh et al. described 10% of severe symptoms, especially in patients with Hyper IgM syndrome.15 Temporary or complete interruption of the infusion is recommended, according to the severity of the reaction.3,4 The safety of the IVIG infusion at our center, with a very low incidence of AEs and no severe reactions, resulted in only 10 (0.6%) complete interruptions during the eight-year follow-up and this may have had a positive impact on the treatment itself, with more stable IgG serum levels, less use of additional drugs to control adverse reactions and, perhaps, a social and economic impact.
Urticaria was the most common reaction, 14 (63.6%) of the infusions having AEs, with almost all (12 out 14) episodes in two patients and complete resolution after changing to the subcutaneous route. The IVIG- related urticaria is not very common, as we observed, and it is usually resultant of IgG aggregates, with resolution after temporary interruption.20,24 Fever has been described as the most common AE in some studies14,20 and, in this study, it was the second most prevalent reaction, followed by headache and nausea/vomiting. Headache is often described in up to 24% of the patients, independent of the age, usually conventional analgesics are effective in controlling symptoms and most cases have resolution in the first 24 hours after stopping the infusion, although some rare patients need to be hospitalized.12,19,24 Headache was seen in three patients and cyproheptadin or acetaminophen was used, as recommended,3,24 to prevent new episodes and, fortunately, none of the patients needed hospital admission.
Several factors have been associated with AEs to IVIG, such as a prior history of infusion reaction, large dose, first infusions and active infection.4,5,7 No significant correlation was observed with age, active infection or first infusions, nor with changing in IVIG preparations in this study, in contrast to previous data.14,15,18-20 The IVIG brand as a factor for AEs has controversial results and, in some studies no association has been found, while, on the other hand, some authors have mentioned a significant incidence of AEs with lyophilized products and preparations with high amounts of sucrose are more related to renal impairment.18,24 In our study, no correlation with IVIG brands was seen, maybe because of the small number of reactions.
It is also important to keep in mind that, in particular cases, as observed in some of our patients, the subcutaneous immunoglobulin (SCIG) should be considered to avoid IG infusion- related AEs.7 In terms of efficacy, the IVIG and SCIG are similar for patients with IEI.1 Potential advantages of SCIG therapy over IVIG include a shorter infusion time, almost no wear-off effects and more stable IG concentrations.1,2,4 Several studies have also demonstrated an overall improvement in the quality of life in patients receiving the SCIG, in comparison to the IVIG administration.1,2,4,7 On the other hand, because of the smaller SCIG doses frequently given, some patients prefer the IVIG administration4 and some authors claim that the SCIG therapy may not be optimal in patients who have poor adherence to treatment.1 Regarding the AEs, the SCIG has significantly lower systemic adverse reactions, which occur in less than 1% of infusions;4 thus, switching from the IVIG to the SCIG seems to be an effective strategy that reduces immunoglobulin-induced AEs, especially for patients who have previously experienced severe AEs or are at high risk of developing adverse effects.2,3 Hydrating the patient before infusions (with careful attention to the volume in patients with comorbidities, such as kidney or heart disease),4 infusing the product at room temperature, monitoring vital signs during infusion, a slower infusion rate in the first administrations and keeping the patient under observation for one hour after the end of the infusion are well-known strategies to reduce AEs related to the IVIG.4,24 Pre-medications are not usually administrated before the IVIG19,24 and some authors claim that it does not influence the frequency of the AEs.3 However, according to data from the Immune Deficiency Foundation (IDF), in a survey of 1500 patients under IVIG treatment, almost half of the patients (45%) stated that they were usually receiving premedication before infusion and, in a retrospective study assessing the safety profile of the IVIG in 424 patients, premedication was provided to 276 patients (65.7%) and the incidence of adverse reactions to the IVIG was significantly lower in patients who received premedication, compared to the non-premedicated patients (18.4% vs. 27.1%, p = 0.04), cited by Cherin et al.24 Our results suggest that premedication has influenced the low rates of AE to IVIG in pediatric patients with IEI, although only a comparative study with and without premedication could give us more convincing data.
ConclusionThis study has some limitations, such as the absence of data on previous reactions to other blood products, detailed data on the use of prophylactic antibiotics, chronic use of steroids to treat comorbidities, or the use of immunosuppressive drugs. Nevertheless, we described a protocol for the IVIG infusion in pediatric patients with IEI who commonly received different brands during their treatment and the low frequency of immediate AEs highlights the safety and tolerability of intravenous immunoglobulin replacement with the procedures established at our center.