During pregnancy, women are at an increased risk of developing iron-deficiency anemia.
ObjectiveThe objective of this study was to assess the diagnostic performance of the reticulocyte hemoglobin equivalent (RET-He) in the early detection of iron-deficiency anemia in a group of pregnant women and to establish a reference range for this parameter in a group of control individuals. Method: A total of 60 patients and 130 control subjects were included in the study. Blood samples collected from the subjects were submitted to a complete blood count and a serum ferritin test and the data were analyzed by comparing the groups and ROC curves.
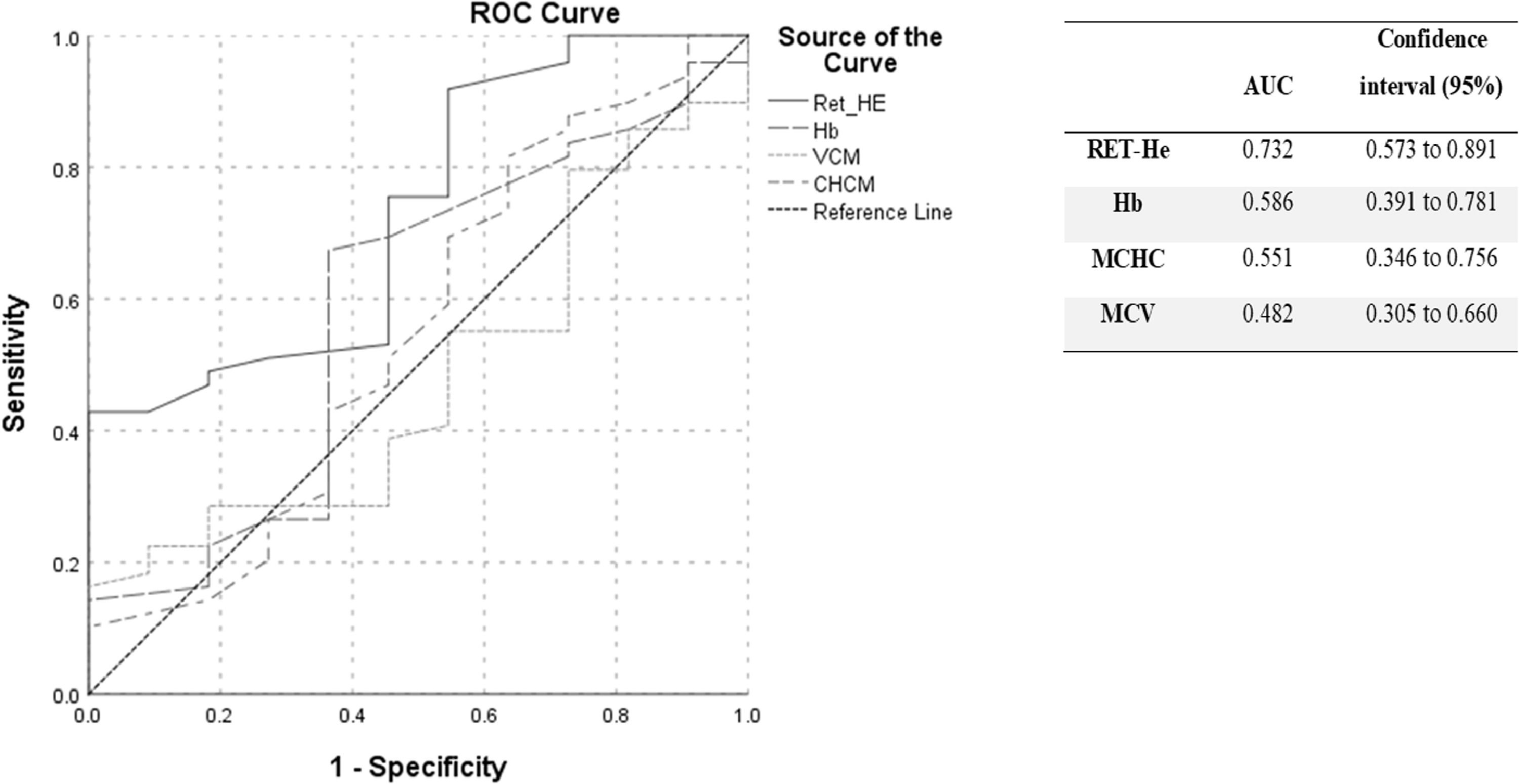
ResultsThe reference range found for the RET-He was between 29.75pg and 38.24pg, with a median of 35pg. The receiver operating characteristic (ROC) curve analysis for the ferritin parameter showed an area under the curve of 0.732 for the RET-He, 0.586 for hemoglobin, 0.551 for the mean corpuscular hemoglobin concentration and 0.482 for the mean corpuscular volume.
ConclusionEarly diagnosis of iron deficiency anemia in pregnancy is essential to prevent damage to both maternal and fetal health. The RET-He presents an excellent potential as an auxiliary tool for the diagnosis of iron deficiency in pregnant women.
Anemia is a condition in which red blood cell numbers, or the hemoglobin concentration inside these cells, is lower than normal. Hemoglobin is needed to carry oxygen and the individual who has too few or abnormal red blood cells, or not enough hemoglobin, the blood will have a decreased capacity to carry oxygen to the body's tissues. Approximately 30% of the world population is estimated to have anemia, with most of these cases being caused by iron deficiency.1 The main laboratory abnormalities observed in this condition are the presence of microcytic and hypochromic erythrocytes, a decrease in hemoglobin levels and a decrease in serum ferritin values.2
Women in the gestational period have a higher risk of presenting with iron-deficiency anemia.3 The anemia may occur due to are inadequate intake of dietary iron, greater fetal demand and hemodilution, a physiological anemia4 common in pregnant women in which changes in the maternal organism, such as changes in blood volume and factors associated with hemostasis, decrease hemoglobin concentration. The blood volume starts to increase from the first trimester, due to the action of the hormones estrogen and progesterone and under the impact of the renin-angiotensin-aldosterone system, constituting a protective and essential mechanism for the provision of a liquid reserve that compensates for the loss of blood during delivery and puerperium. The limited modulation of the circulating hemoglobin, resulting from pregnancy adaptations, does not cause damage to the maternal-fetal binomial5,6 and the reference value for the measurement of hemoglobin in pregnancy is therefore different from that for non-pregnant women. Although iron absorption is moderately high during pregnancy, the amount of iron absorbed by the diet, along with the mobilization of stored iron, is generally insufficient to supply the demand imposed by pregnancy. At the beginning of pregnancy (first trimester, or up to 14 weeks) there is an increase in serum iron and ferritin, probably due to the still small demand of the initial pregnancy, as well as the positive iron balance.6 Iron deficiency is associated with a higher rate of mother/fetus morbidity and mortality and the most common complications are early labor, low birth weight, preeclampsia and a higher risk for miscarriage. However, the etiology of anemia during pregnancy in developing countries also includes factors, such as low socioeconomic and education levels and high parity.4 Furthermore, anemia impairs mental development and the ability to work and study productively.
During the course of pregnancy, there is a progressive fall in the mean values of hemoglobin, MCV and ferritin, but the increased demand for iron during pregnancy is supplied by the increase in the absorption of iron from the appropriate diet in the same period.6
According to the World Health Organization (WHO), the reference value for hemoglobin for non-pregnant women is 120 g/L or higher, while the reference value for normal pregnant women is 110 g/L.7 The Centers for Disease Control and Prevention (CDC) recommends an upper normal level of 110 g/L during the first and third trimesters and an upper level of 105 g/L in the second trimester.8 In Brazil, the Ministry of Health determines an upper hemoglobin level of 110 g/L for pregnant women.3
The clinical routine for assessing anemia in pregnant women includes measuring the hemoglobin (Hb) concentration,9 mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), reticulocyte count10 and other biomarkers for determining iron status, such as serum ferritin (SF), soluble transferrin receptor (sTfR), zinc protoporphyrin, serum iron and hepcidin, as well as total iron-binding capacity or transferrin saturation (TSAT).11–13 Serum ferritin is the most clinically applicable parameter in pregnancy,3,10 however serum ferritin values can be affected by other non-physiological changes, such as the presence of inflammatory and infectious processes, which increase ferritin levels,14,15 potentially limiting this parameter in certain cases.
The study and improvement of new laboratory markers, such as the reticulocyte parameters provided by some equipment, must allow for the earliest possible diagnosis of anemia. Standardizing and implementing these new parameters is essential to fully assess biomarkers and their clinical and laboratoial correlations. Erythropoiesis is traditionally monitored by counting the number of reticulocytes in the peripheral blood, since decreases in circulating reticulocyte numbers are indicative of lower bone marrow activity.16 The reticulocyte hemoglobin equivalent (RET-He) is a parameter that reflects the hemoglobin content in reticulocytes (CHr). Since the normal lifespan of these cells is between one and two days in the peripheral blood, RET-He is a good indicator of the availability of iron and can point to early iron-deficient erythropoiesis.16–18 The RET-He marker is also useful in monitoring the response to iron-replacement therapy and, due to the short circulation time of reticulocytes in the peripheral blood, may help in monitoring the bone marrow response soon after the start of treatment.16,19
Considering the risks incurred by iron deficiency anemia to both the mother and the developing baby, early detection of iron deficiency in pregnant patients is important. Studies have shown that the hemoglobin concentration in reticulocytes is a good parameter for detecting anemia.14,17,18,20–22
ObjectiveThe objective of this study was to assess the RET-He parameter as a possible marker for the early diagnosis of iron deficiency anemia in patients in different gestational periods. Additionally, we aimed to establish a range of normality for RET-He in healthy individuals.
MethodsThis study was approved by the Hospital de Clínicas de Porto Alegre Research Ethics Committee (protocol no. 170328). All participants signed an informed consent term. To determine the reference range for the RET-He, control samples were collected (in K3-EDTA) from 130 donors (65 men and 65 women) at the Hospital de Clínicas de Porto Alegre Blood Bank. We performed the complete blood and automated reticulocyte counts and the reticulocyte subpopulation analysis. The serum ferritin was also evaluated in women. Hemoglobin with a concentration below 110 g/L and ferritin below 13 ng/mL were established as the criteria for anemia.
For the group of pregnant women, 60 blood samples were collected (in K3-EDTA) from pregnant women who had undergone prenatal examinations at the Pharmacy School Clinical Analysis Laboratory at the Universidade Federal do Rio Grande do Sul from August to October 2017. The pregnant women were divided into three groups, based on the gestational period. We performed the complete blood and automated reticulocyte counts and the reticulocyte subpopulation analysis. Ferritin was also measured in the samples obtained. For comparison with the group of pregnant women, samples from 65 women belonging to the blood bank donor group were used.
Blood and reticulocyte counts were performed at the Hospital de Clínicas Laboratory Diagnosis Service Clinical Biochemistry Unit in Porto Alegre, Brazil, using the Sysmex XN10 Roche® hematology system (Sysmex Corporation, Kobe, Japan). The reticulocyte channel uses fluorescence flow cytometry technology for the reticulocyte and erythrocyte counts and the immature reticulocyte fraction (IRF) and RET-He measurements. Samples were processed no more than four hours after collection.
Ferritin levels were measured at the Universidade Federal do Rio Grande do Sul Pharmacy School using the COBAS 411 equipment and electrochemiluminescence method. For the ferritin dosage, plasma was stored frozen (−20 °C) from the day of collection until the time of analysis.
The sample size was calculated using the WINPEPI software, version 11.44. The number of controls was based on the recommendations by the Clinical and Laboratory Standards Institute and the International Federation of Clinical Chemistry.23,24 Statistical analysis of the results was performed using the SPSS software, version 18.0. We performed a descriptive analysis of the data. The comparison between men and women in the control group was carried out using the Mann-Whitney U test. Kruskal-Wallis tests (non-parametric variables) and multiple comparisons were made with Bonferroni's adjustments. The ANOVA (parametric variables) was used to compare the variables between groups. The RET-He was assessed using a Receiver Operating Characteristic (ROC) curve and then compared to ferritin (reference range of 13––150 ng/mL), the gold standard for the diagnosis of iron-deficiency anemia.
ResultsBlood samples from a total of 130 control subjects were analyzed. Of these subjects, 65 were men and 65 were women and their median age was 36.0 years (16–66 years). Sixty samples from pregnant women were evaluated (20 in each gestational period); their median age was 26.5 years (17–41 years).
Hemoglobin and/or ferritin concentrations below normal were found in 2 (two) pregnant women in the 1st trimester with anemia, 5 (five) in the 2nd trimester and 11 (eleven) in the 3rd trimester.
Median RET-He values for the control group were 35.20pg for men and 35.00pg for women (p = 0.310). Based on these data, the confidence interval was calculated, covering all subjects in the control group. The median value of the RET-He for men and women was 35.00pg. The reference intervals for the RET-He were calculated, using the 2.5% and 97.5% percentiles, as in a range of between 29.75pg and 38.24pg.
The correlation between the IRF and RET-He values in the total group of pregnant women was rS = −0.295 (p = 0.022).
Table 1 shows the median (25% and 75% percentiles) values for the parameters analyzed in each group. For the comparative study, only values for female controls were used. Figure 1 shows the ROC, the area under the curve (AUC) and their respective confidence intervals (95%), for the RET-He, Hb, MCHC and MCV parameters. Table 2 presents the cut-off points for RET-He and their respective specificities and sensitivities. According to the data obtained, the chosen value was 35.55, which presented the best specificity, with maximum sensitivity.
Ferritin and hematological parameters in pregnant women, according to the gestational period, compared to the control group.
RBC: red blood cell count; Hb: hemoglobin; MCV: mean corpuscular volume; MCHC: mean corpuscular hemoglobin concentration; RET-rel: relative reticulocyte count; RET-abs: absolute reticulocyte count; IRF: immature reticulocyte fraction; RET-He: hemoglobin content in reticulocytes; SD: standard deviation; p < 0.05: significance.
The letters (a, b and c) represent the comparison between the groups by the Bolferroni adjustment; similar letters indicate that groups do not differ statistically.
The diagnostic accuracy for the RET-He iron deficiency has been evaluated by several authors. This parameter has been shown to have great potential as a test for detecting the early stages of iron-deficiency anemia14,17,18,20–22 and for the follow-up of replacement therapy.19–21 Reference intervals for the RET-He have already been determined by other authors.17,22,25,26 The range found in the present study, that of between 29.75pg and 38.24pg, is consistent with the range established by Scherer et al., who proposed a range of 30.30pg to 36.96pg for a group of healthy patients.26 Furthermore, the range determined is also in accordance with a report by Levy et al., who proposed a 31.2-pg cut-off value to distinguish iron deficiency from non-iron deficiency.27
Data in Table 1 indicate that the RET-He showed a pattern similar to that of serum ferritin, with a significant difference in the 3rd trimester of pregnancy, in relation to the control groups and the 1st and 2nd trimesters. It was expected, as the number of women with ferritin below the normal range is higher in the second and particularly in third semester.28
With regard to the IRF, there was a significant difference between the pregnant and control groups and between pregnant women in the 1st and 3rd trimesters. According to the studies by Choi et al. in 2001, erythropoietic activity increases with gestational age in pregnant women and returns to normal five weeks after delivery. They observed that the level of the sTfR is closely related to the reticulocyte maturity index (RMI) and subpopulations of reticulocytes and can be used to monitor erythropoiesis during pregnancy. When pregnant women with evidence of anemia or iron deficiency were excluded, there were no significant differences in the level of serum ferritin between the first and the third trimesters. However, there was an increase in the reticulocyte subpopulations, in particular the RMI, during the second and third quarters.28
In this study, the RET-He was found to be the best marker for detecting anemia in pregnant women, when compared to the other parameters determined (Hb, MCV and MCHC), consistent with previous studies demonstrating that the RET-He was an excellent parameter for diagnosing iron deficiency. Ervasti et al. evaluated the accuracy of new markers, including the hemoglobin content in reticulocytes, using the Advia® equipment, for the diagnosis of iron deficiency in women soon after delivery,15 and reported that the RET-He marker presented an AUC of 0.79 and performed well, compared to other traditional parameters, including Hb, MCV, MCHC and ferritin. These findings corroborate the results of our study, which found an AUC of 0.73.
Brugnara et al. assessed the performance of the RET-He in patients chronically treated with hemodialysis. The authors concluded that the RET-He presented excellent results (AUC = 0.913), in comparison to parameters traditionally used for diagnosing iron-deficiency anemia.22 Dalimunthe et al. also investigated the use of the RET-He in patients undergoing hemodialysis and observed a good diagnostic performance (AUC = 0.818). 21 These authors also evaluated the RET-He as an early marker of response to iron supplementation and obtained good results. In another study, Levy et al. analyzed RET-He as a parameter for the diagnosis of subclinical iron deficiency in pregnant patients, also considering the RET-He a reliable marker (AUC = 0.81), even when compared with standard tests.27
Ervarsti et al. determined a cut-off point of 31.9pg for the RET-He, with a sensitivity of 80.7% and a specificity of 71.3%,15 which were similar to values found in other studies.17,20,21 In our study, we observed an excellent specificity (93.9%), but a low sensitivity (34.6%), using the same cut-off point. At 35.25pg, we obtained the highest possible sensitivity (100%), but low (42.9%) specificity. One of the factors leading to this discrepancy may be the difference in sample sizes. Other factors, such as the reduced number of anemic pregnant women in the population studied and the interference of biological factors in ferritin dosage, may have also influenced the results. The decision regarding the best cut-off point should prioritize a parameter with high sensitivity to detect iron deficiency early.
The World Health Organization recommends iron supplementation for pregnant women throughout the gestational period.29 Similarly, in Brazil, the National Iron Supplementation Program recommends prophylactic iron supplementation as part of prenatal care during pregnancy.9 Of the 60 pregnant women evaluated in the present study, 35% reported having used ferrous sulfate at some point in the gestational period, which may have interfered with the observation of the development of iron-deficiency anemia in this population. Another potentially interfering factor is the fact that ferritin acts as an acute-phase reagent14 and is increased in the presence of inflammatory and infectious processes. As such, there is an increase in the levels of this marker in the month before childbirth,5 which may mask the presence of iron-deficiency anemia during this period. Likewise, the occurrence of inflammatory processes and infections in the gestational period may also have interfered with the observation of iron deficiency via ferritin dosage, as these data were not assessed in our study. Despite all the limitations of ferritin determination, guidelines and clinical practices indicate that it is an important marker of anemia in pregnant women.
In addition to demonstrating a better performance than the traditional markers for the diagnosis of iron-deficiency anemia14,17,18,20–22 and efficiency for the follow-up of patients undergoing iron supplementation treatment,19–21 the RET-He has great potential as an auxiliary tool during the follow-up of pregnant women in prenatal care. The progressive decrease of a patient's RET-He values may indicate the onset of iron deficiency, indicating a need for further investigation, using traditional iron dosages. The monitoring of iron-deficiency anemia in pregnant women is suggested, during complete blood count analysis, in the established prenatal protocol of the Brazilian Ministry of Health.30 As such, since the RET-He is included in the complete blood count analysis of the equipment that performs reticulocyte testing, this is a low-cost alternative that does not require additional sample collection, representing an advantage over other traditional markers.
Among the limitations of our study, the impossibility of performing a survey of pregnant women who were receiving oral iron supplementation should be noted. Additional studies are needed that include a larger number of anemic pregnant women in different gestational periods to better understand the role of the RET-He in iron deficiency anemia in pregnant women and its importance as an early marker. It is important to note that the results obtained are relevant to the equipment used in this study and that other equipment or methodologies require further studies.
ConclusionThe early diagnosis of iron deficiency anemia in pregnancy is essential to prevent damage to both maternal and fetal health. The RET-He presents potential as an excellent alternative auxiliary tool for the diagnosis of iron deficiency in pregnant women, especially during prenatal care. Integrating periodic observations of RET-He values into routine blood counts may facilitate monitoring for the early development of anemia, where a progressive decrease in RET-He values could indicate the need for further investigation, using conventional iron dosages.
Financial supportThis study was supported by the Hospital de Clínicas de Porto Alegre Research and Event Incentive Fund (FIPE-HCPA), under Grant number 2017-0328.