Phagocytosis of autoantibody-sensitized coated platelets through Fc gamma receptors on phagocytic cells is an important mechanism of thrombocytopenia in primary immune thrombocytopenia (ITP).
ObjectiveWe aimed to investigate the contribution of the FcγRIIa and FcγRIIIa genes polymorphism to the risk of ITP and their association with disease characteristics in Egyptian children.
MethodsA case control study was conducted on eighty children with primary ITP and eighty age and sex healthy matched subjects as a control group. The FcγRIIa and FcγRIIIa genes polymorphism was detected using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP).
ResultsWe found that the FcγRIIa‐131H and ‐131R allele frequencies were 51.3 % and 48.7%, respectively, in children with ITP, versus 75% and 25%, respectively, in controls (p = 0.002). The compound heterozygous HR genotype was significantly higher in ITP patients (p < 0.05). The FcγRIIIa-158F and ‐158V allele frequencies were 46.3% and 53.7%, respectively, in children with ITP, versus 70% and 30%, respectively, in controls (p = 0.002). The compound heterozygous VF genotype was significantly higher in ITP patients (p < 0.05). The combined HR/FV genotype was 47.5% in ITP patients, versus 10% in controls (p < 0.001). No significant difference was found between children with newly diagnosed ITP and those who developed chronic ITP, regarding the frequency distribution of the FcγRIIa and FcγRIIIa alleles and genotypes (p > 0.05).
ConclusionThere is a possible association of the FcγRIIa and FcγRIIIa genes polymorphism with the risk for, and genetic susceptibility to ITP in Egyptian children, but large-scale studies are still needed to support our findings.
The hallmark of childhood primary immune thrombocytopenia (ITP) is isolated thrombocytopenia and platelet count < 100,000/µL, with normal hemoglobin and white blood cell counts. The ITP was classified as newly diagnosed ITP (from diagnosis up to 3 months), persistent ITP (from 3 months up to 12 months from diagnosis) and chronic ITP (the presence of ITP for more than 12 months from diagnosis).1
The etiology of ITP remains unknown in most cases, however viral infection and other environmental factors were suggested to be an important trigger in this disease.2,3 Furthermore, complex dysregulation of the immune system is implicated in the pathogenesis of this disease.4
The presence of autoantibodies that target platelet membrane glycoproteins (GP) Ib, GPIIb/IIIa or GPVI and the clearance of autoantibodies-coated platelets by phagocytic cells are thought to be one of the most important factors contributing to thrombocytopenia in ITP.5 Autoantibodies bind to the platelet-specific membrane antigen through their Fab portions. Because of this binding, platelets are phagocytosed through the Fc portion of these autoantibodies by phagocytic cell monocytes and macrophages which express Fc receptors through the antibody-dependent cell-mediated cytotoxicity function in the spleen and liver.6
Three different families among those of the FcγR differ in their IgG affinity, preferences for IgG subclasses, expression patterns and the intracellular signals they elicit in the cell. Additionally, the presence of well-characterized genetic polymorphisms contribute to the FcγR heterogeneity.7 Single nucleotide polymorphisms are among the genetic factors causing a series of changes in the human genome and leading to the development of autoimmune disease, including ITP.8
This study was conducted to investigate the contribution of the FcγRIIa and FcγRIIIa to the risk of ITP and its association with disease characteristics in Egyptian children.
MethodsThis case control study enrolled eighty patients with primary immune thrombocytopenia and eighty sex- and age-matched healthy children as a control group. Healthy children were recruited from the outpatient pediatric clinic during visits of their relatives to the clinic and they were not among those related to diseased patients. Children with primary immune thrombocytopenia were admitted and placed on a regular follow-up schedule for approximately one year at the Pediatric Hematology Unit, Zagazig University Hospital and Medical Biochemistry Department, from June 2018 until June 2019.
Inclusion criteria: approval to sign an informed written consent, patients with newly diagnosed ITP, patients aged > 1 year and < 18 years and both sexes were included.
Exclusion criteria: refusal to sign an informed written consent, patients with persistent ITP, patients with chronic ITP, patients with secondary immune thrombocytopenia and patients aged < 1 year or > 18 years.
Definitions
The ITP was defined by the International Working Group as a platelet count less than 100 × 109/L in the absence of other disorders or causes that may be associated with thrombocytopenia. The ITP was classified as newly diagnosed ITP (from diagnosis up to 3 months), persistent ITP (from 3 months up to 12 months from diagnosis) and chronic ITP (the presence of ITP for more than 12 months from diagnosis).1
All enrolled children were subjected to a complete general physical examination, as well as routine investigations in the form of the complete blood count at diagnosis. The genotyping of the FcγRIIa and FcγRIIIa genes polymorphism was performed with the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) for patients and controls.
Blood sampling and DNA extractionTwo-milliliter whole blood samples were drawn from all participants and were collected into tubes containing K-EDTA for genomic DNA extraction using the spin column method according to the manufacturer protocol of the Thermo Scientific™ Gene JET™ DNA purification kits (Thermo Scientific™ Biotechnology, Seongnam-Si, Korea, catalog number K0721). The DNA extracts were stored at -20°C until the time of use.
Genotyping of FcγRIIa 131 H/R (rs1801274) gene by polymerase chain reaction followed by restriction fragment length polymorphism (PCR-RFLP) analysisThe extracted DNA was amplified by the polymerase chain reaction (PCR) using the recombinant Taq polymerase master mix (Dream Taq Tm green pc, code number k1081, lot no. 00643300) (Thermo Fisher scientific Ballics UAB, V A Craciuno 8, LT-002241Vilnius, Lithuania). PCR reactions were performed in the 25-μl volume comprised of 12.5μl of Taq PCR master mix (0.1 unit/μl Taq DNA polymerase, 32mM of (NH4)2 SO4, 130mM of Tris HCl, 5.5mM MgCl2 and 0.4mM of each dNTP), 5.5μl of distilled water, 5μl of template DNA and 1μl (25pmol) of each primer (Fermentas–Lithuania), forward 5´-GGA AAA TCC CAG AAA TTC TCG C-3´ and reverse 5´-CAA CAG CCT GAC TAC CTA TTA CGC GGG-3´.
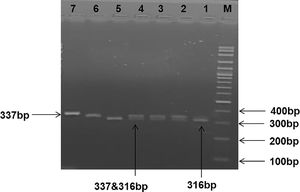
The gene amplification was performed according to the following PCR protocol: 94°C for 3 minutes, followed by 35 cycles at 94°C for 15 seconds, 55°C for 30 seconds and 72°C for 40 seconds. A final extension stage was performed at 72°C for 7 min. The amplified PCR product was then digested with 20U of the restriction enzyme BstUI restriction endonuclease (Thermo Fissure – Lithuania). Digested products were electrophoresed in a 2.5% agarose gel. DNA fragments were visualized by ethidium bromide. The FCGR2A wild type H/H individuals yield a 337-bp fragment, whereas the mutant R/R yields two fragments of 316 and 21bp. The heterozygous mutation (H/R genotype) yields three bands of 337, 316 and 21bp (Figure 1).
Gel electrophoresis picture showing RFLP analysis of FCGR2A gene polymorphisms. Lane 1: DNA marker (100–1500 bps). Lanes 1 and 5 show mutant FCGR2A homozygous genotype R/R (316 bp). Lanes 2, 3 and 4 show heterozygous H/R FCGR2A genotype (337, 316 bp). Lanes 6 and 7 show wild FCGR2A genotype H/H (337 bp).
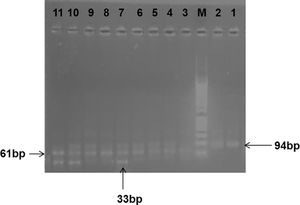
The genotyping of the FcgR3A- was performed according to Koene et al.9 The first pair of primers, forward 5´-ATA TTT ACA GAA TGG CAC AGG-3´ and 5´-GAC TTG GTA CCC AGG TTG AA-3´ (Fermentas–Lithuania), were used to amplify a 1.2-kb fragment containing the polymorphic site. The Taq polymerase master mix (Dream Taq Tm green pc, code number k1081, lot no. 00643300) (Thermo Fisher scientific Ballics UAB, V A Craciuno 8, LT-002241Vilnius, Lithuania). PCR reactions were performed in the 50-μl volume comprised of 25μl of Taq PCR master mix (Dream Taq Tm green pc, code number k1081, lot no. 00643300), 11μl of distilled water, 10μl of template DNA and 2μl (25pmol) of each primer. The first PCR cycle consisted of a 10-min. denaturation at 95°C, a 1.5-min. primer annealing at 56°C and a 1.5-min. extension 72°C. This was followed by 35 cycles in which the denaturing time was decreased to 1min. The last cycle was followed by 8 min. at 72°C to complete the extension. In the second PCR run, another pair of primers were used, forward 5´-ATC AGA TTC GAT 158V/F CCT ACT TCT GCA GGG GGC AT-3´ and reverse 5´-ACG TGC TGA GCT TGA GTG ATG GTG ATG TTC AC-3´ This “nested” PCR was performed with 5μl of the amplified fragment, 1μl of each primer, 12.5μl of the master mix and 5.5μl of distilled water. The first cycle consisted of a 5-min. denaturing at 95°C, a 1-min. primer annealing at 64°C, and a 1-min. extension at 72°C. This was followed by 35 cycles in which the denaturing time was 1 min. The last cycle was followed by 9.5min. at 72°C to complete the extension. The 94-bp PCR product was digested with NIaIII (Thermo Fissure – Lithuania) and the digested fragments were electrophoresed in a 5% agarose gel stained with ethidium bromide and visualized with UV light. The normal FcγRIIIa wild type (F/F genotype) yields non-digested 94bp, while homozygous mutant FcγRIIIa (V/V genotype) yields two bands of 61 and 33bp. The heterozygous mutation (F/V genotype) yields three bands of 94, 61 and 33bp (Figure 2).
Gel electrophoresis picture showing RFLP analysis of FCGR3A gene polymorphisms. Lanes 1 and 2 show wild FCGR3A genotype F/F (94 bp), Lane M: DNA marker (50–1000 bps). Lanes 3, 8, 9 and 11 show mutant FCGR3A homozygous genotype V/V (61 and 33 bp). Lanes 4, 5, 6, 7 and 10 show heterozygous F/V FCGR3A genotype (94, 61 and 33 bp).
The study was carried out according to the ethical standards of the declaration of Helsinki 1964, that was revised in 2000, and it was approved by our local ethical committee and the Zigazig University Faculty of Medicine. Informed written consent, in addition to an assent form, were obtained from the study participants and/or their guardians.
Statistical analysisData handling and adequate statistical analysis were performed using the SPSS version 19 software (bpPSS Inc., IBM Crop. Armonk, N.Y, USA). Quantitative variables were presented as mean ± standard deviation. When comparing quantitative data, we used the Student's t-test and one-way ANOVA. The Hardy-Weinberg equilibrium was tested by the Chi-square test to check for three genotypes among the studied groups. The 2 × 2 contingency table was used to analyze the categorical data by using the Fisher's exact test. The odds ratio calculation with a 95% confidence interval was used to assess the strength of genetic risk among patients and controls. A value was interpreted as statistically significant when the p-value was < 0.05.
ResultsA total of eighty children (48 males and 32 females) diagnosed with ITP and eighty sex- and age-matched healthy subjects were included in this study. Children diagnosed with ITP were followed up at least for one year. Their mean age was 5.8 years, ranging from 3 to 15 years (Table 1).
Demographic data of the studied groups.
t: independent t-test. X2: chi-square test.
The mean initial platelet count among our patients was 22 × 103/uL versus 331 × 103/uL in controls (p < 0.05), while the mean initial hemoglobin level was 10.6g/dL in patients versus 10.3g/dl in controls (p > 0.05) (Table 2).
Laboratory data of the studied groups.
t: independent t-test.
The mean age of patients at diagnosis was 4.51 years. Purpura, ecchymosis and external bleeding were present in 64 (80%), 52 (65%) and 45 (56%) of our patients, respectively. We did not find any severe or life-threatening bleeding in our patients. Preceding viral infection was detected in 42 out of 69 (61%) of the patients with acute ITP and in 4 (21%) out of 19 patients with chronic ITP (p < 0.001).
Fourteen patients (17.5%) were subjected to the observation strategy and did not receive treatment, while 48 patients (60%) received corticosteroids, 14 (17.5%) received combined corticosteroids and intravenous immunoglobulins and 4 (5%) received only intravenous immunoglobulins as a first-line therapy.
The distribution frequency of FcγRIIa alleles in children with ITP is significantly different from that in controls. The FcγRIIa‐131H and ‐131R frequencies were 51.3% and 48.7%, respectively, in children with ITP versus 75% and 25%, respectively, in controls (odds ratio (OR)) [95% confidence interval (95%CI)]: 0.35 [0.18 - 0.68] for the H allele and 2.85 [1.46 - 5.57] for the R allele, p = 0.002).
Regarding the frequency distribution of FcγRIIa genotypes, a significant difference was found between children with ITP and controls, homozygous H (HH), homozygous R (RR) and compound heterozygous HR genotypes being present in 22.5%, 20% and 57.5%, respectively, in children with ITP, versus 70%, 20% and 10%, respectively, in controls.
The frequency distribution of FcγRIIIa alleles in children with ITP is significantly different from that in controls. The FcγRIIIa-158F and ‐158V frequencies were 46.3% and 53.7%, respectively, in children with ITP, versus 70% and 30%, respectively, in controls (OR [95%CI]: 0.37 [0.19 - 0.71] for the F allele and 2.7 [1.41 - 5.19] for the V allele, p = 0.002).
The frequency of the three FcγRIIIa genotypes, FF, VF and VV, in children with ITP patients was 10%, 72.5% and 17.5%, respectively, versus 50%, 40% and 10%, respectively, in controls (p = 0.001).
The frequency distribution of individual FcγRIIa and FcγRIIIa alleles and genotypes is displayed in Table 3.
Frequency of FcγRIIa and FcγRIIIa genotypes among the groups studied.
OR: odds ratio.
The combined HR/FV genotype was found to be more prevalent in ITP patients, compared to controls (47.5% versus 10%, respectively, p < 0.001). The frequency distribution of the combined FcγRIIa and FcγRIIIa genotypes is displayed in Table 4.
Analysis of combined genotypes of FcγRIIa and FcγRIIIa among the groups studied.
OR: odds ratio.
Our patients were followed up for at least for one year. The mean follow-up period was 1.4 years, ranging from 1 to 1.8 years. During the follow-up period, 19 (23.8%) children with ITP developed chronic disease.
There was no significant difference between patients with newly diagnosed ITP and those with chronic ITP, regarding the frequency distribution of the FcγRIIa and FcγRIIIa alleles and genotypes (p > 0.05) (Table 5).
Relationship between different FcγRIIa and FcγRIIIa genotypes and disease chronicity.
OR: odds ratio.
The human Fcγ receptors family comprises glycoproteins which, through binding to the Fc portion of the IgG, are involved in the preservation of antibodies. Various studies reported that the genetic alteration of the FcγRIIa and /or FcγRIIIa might result in the alteration of the receptor-binding affinity with the Igs.7,10,11
Fcγ receptor polymorphisms have been associated with different diseases, such as heparin-induced thrombocytopenia11 and systemic lupus erythematosus (SLE),12 and correlated with the clinical response to rituximab-based treatment in patients with follicular lymphoma.13 Fcγ receptor polymorphism may be implicated in disease occurrence, clinical presentation and disease severity, as well as the outcomes.
In the current study, we investigated the contribution of the FcγRIIa and FcγRIIIa genes polymorphism to the risk of ITP and its association with disease characteristics and we found a significant difference in genotype distribution and allele frequencies for the FcγRIIa and FcγRIIIa polymorphism in ITP patients versus controls (p < 0.05), the FcγRIIa‐131H/H and compound heterozygous FcγRIIIa-158 V/F genotypes being the most present genotypes among ITP patients (p < 0.05).
Similarly, Carcao et al.14 found that the genotypic frequencies for the FcγRIIa-131H and -131R and the FcγRIIIa-158F and -158V in children with ITP were significantly different, compared to the control group, suggesting that the inheritance of these receptor polymorphisms may play a significant role in the development of ITP in children.
Our findings were matched with a study conducted by Foster et al.,15 who reported that the genotypic frequencies among ITP patients for the FcγRIIa-131R/R:H/R:H/H were 19%:36%:44% and for FcγRIIIa-158F/F:V/F:V/V, 35%:62%:3%.
Our results regarding the association of FcγRIIIa V/F SNP and ITP conformed to the results of Papagianni et al.,16 which implicated that the heterozygous (VF) genotype was significantly higher in ITP children, compared to controls.
In addition, Mohamed et al.17 documented the frequency distribution of ITP patients who have inherited the heterozygous FcγRIIIa VF genotype was higher, compared with healthy controls (p = 0.005). However, they did not detect a significant difference in allele frequencies of the FcγRIIIa V/F gene polymorphism in ITP patients and healthy controls (p = 0.172) and concluded that the inheritance of the heterozygous (VF) genotype may have a role in the development of ITP.
Similarly, the meta-analysis conducted by Xu et al.18 reported that the FcγRIIIa 158F>V polymorphism had a strong relationship with ITP susceptibility, regarding the five genetic models studied (p < 0.05). However, no similar relationship was found between the FcγRIIa-131H>R polymorphism and ITP susceptibility (p > 0.05).
Furthermore, Eyada et al.,10 in their study on pediatric ITP patients, reported that the V allele and FcγRIIIa FV heterotype were significantly higher in patients with ITP, with a notable increase in ITP risk (OR = 1.96 and 2.55, respectively, p < 0.05) .
Pavkovic et al.19 supported our findings by documenting a remarkably higher frequency of the high-affinity FcγRIIIa -158V allele in patients with ITP, compared to the control subjects (47.2% versus 37.5%; p = 0.037), but in contrast, they did not find a significant difference in the genotype distribution or allele frequencies for the FcγRIIa -131H/R between patients and controls, p = 0.652 and p = 0.478, respectively.
Our results were different from those reported by Williams et al.20 and Joutsi et al.,21 who found that the FcγRIIa-131R allele was predominant in adults with refractory ITP. They attributed this to the decreased affinity of the FcγRIIa-131R for IgG2, which subsequently results in a reduced or incomplete clearance of antibody–infectious agent complexes, leading to a predisposition to ITP development. The controversy may be attributed to the difference in the age of patients in the two studies.
On the contrary, Mohamed et al.17 studied the role of polymorphism in the FcγRIIa R/H gene and the development of childhood ITP and found that it is not related to ITP susceptibility.
Moreover, our results were not matched with Papagianni et al.,16 who reported no significant differences between children with ITP and controls, regarding the frequency distribution of the FcγRIIa-131H and -131R and the FcγRIIIa-158F and -158V in children with ITP.
On the contrary, Horsewood et al.22 and Fujimoto et al.23 found no significant difference between children with ITP and controls, regarding the genotype distribution and allele frequencies for the FcγRIIa and FcγRIIIa polymorphisms.
In addition, our results were inconsistent with those reported by Kuhne, 24 who did not detect a significant association regarding the frequency distribution of the FcγRIIIa V/F SNP and the development of ITP.
Based on the fact that combining different polymorphic variants may have a synergistic effect, we compared patients who had the combination of the FcγRIIa-131H and -131R and the FcγRIIIa-158F and -158V genotypes with the controls and we found that the combined HR/FV genotype was more common in children with ITP, compared to controls (47.5% versus 10%, p < 0.001).
Similarly, Pavkovic et al.19 compared the allelic frequency of the FcγRIIa and FcγRIIIa in ITP patients and controls and reported that the combination of the FcγRIIa -131H and FcγRIIIa -158V alleles was more common in patients with ITP than in the controls (55% versus 40%, p = 0.024). Additionally, the combination of the low-affinity alleles FcγRIIa -131R and FcγRIIIa -158F was less common in patients with ITP than in the controls (50.4% versus 70%, p = 0.027). This observation has its limitation because the authors could not know whether the increase in the high-affinity alleles for both polymorphisms is caused by the more common appearance of the V allele of the FcγRIIIa in addition to the limited number of cases studied.
Furthermore, Carcao et al.14 found in their study that the combination of the FcγRIIa-131H and FcγRIIIa-158V alleles was more common in patients with ITP than in the controls (61% versus 46%, p = 0.02). The corollary to this was that the combination of the lower affinity alleles (FcγRIIa-131R and FcγRIIIa-158F) was less common in patients with ITP, compared to controls (54% versus 70%, p = 0.02).
However, Amorim et al.25 did not find a significant difference between the ITP group and controls regarding the analysis of the combinations of alleles of the high-affinity Fc receptor, but reported that ITP individuals with this combination had a shorter duration of disease (p = 0.01).
In the current study, we did not find a significant difference between children with newly diagnosed ITP and those who later developed chronic ITP, regarding the frequency distribution of the FcγRIIa and FcγRIIIa alleles and genotypes (p > 0.05).
Similarly, Carcao et al.14 found no statistical difference between children who later developed chronic ITP, compared to children with acute ITP, regarding the frequency distribution of the FcγRIIa and FcγRIIIa alleles and genotypes, suggesting that additional factors are responsible for the progression of the disease to its chronic form. This observation underscores the importance of the Fcγ receptor-mediated cell clearance in childhood ITP.
Different results were obtained by Foster et al.,15 who conducted their study on chronic ITP patients versus controls and reported that the heterozygous V/F genotype of the FcγRIIIa was increased in chronic ITP patients, compared to controls (62% versus 41%, p = 0.017, OR = 2.38; 95% CI 1.09 – 5.32), while the FcγRIIa allele, R131, was observed in 7 out of 36 (19%) chronic ITP patients and in 54 out of 218 (25%) controls (p = 0.49). This discrepancy may be due to the difference in the study population.
On the contrary, the homozygous RR genotype of the FcγRIIa was reported to be increased in adults with chronic ITP (48% versus 18%, p = 0.005).20
Two previous Egyptian studies investigated the nucleotide polymorphism in the FcγRIIa and FcγRIIIa and its association with the incidence of childhood primary immune thrombocytopenia. Eyada et al.10 reported that the frequency of the FcγRIIa H allele was significantly higher among chronic ITP patients. Mohamed et al.17 did not investigate the association between the frequency of different alleles and disease chronicity.
A very crucial limitation in the two previous Egyptian studies is that they included two different cohorts: patients with acute ITP and patients with chronic ITP. The results would be more reliable if they included a single cohort at their first presentation and followed this cohort to show if they would develop chronic disease or not. In our study, we tried to overcome this limitation and included a single cohort at their first presentation.
It is notable that the course of chronic ITP varies in children from that found in the adult population, the main difference being the higher potential for spontaneous remission in children.
This study supports the hypothesis that genetic factors contribute to the pathogenesis of childhood ITP.
ConclusionOur results suggested a possible role of the FcγRIIa and FcγRIIIa genetic polymorphism in the risk and genetic susceptibility to ITP in Egyptian children, but further larger prospective studies are still needed to support our findings.
Limitation and future researchThe main limitation in our research is small sample size and financial restrictions that made us unable to recruite more participants. Large scale, multicenter studies are still needed to support our findings.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors thank the study participants for their unrestricted cooperation.