Diffuse large B-cell lymphoma (DLBCL) treatment in older patients is challenging. The Determination and Management of Risks for Practices and Procedures in the Elderly (DRIPP) is a multidimensional evaluation program that involves patients undergoing oncological treatments.
ObjectiveWe aimed to evaluate the overall survival and progression-free survival (PFS) of patients evaluated and those not evaluated by the DRIPP.
Materials and methodsRetrospective cohort study, patients > 65 years with DLBCL were included. They were divided into 3 groups: patients with a diagnosis prior to the DRIPP implementation (pre-DRIPP), patients with the DRIPP (DRIPP) and patients with a diagnosis after the DRIPP implementation, but who did not undergo the evaluation (non-DRIPP).
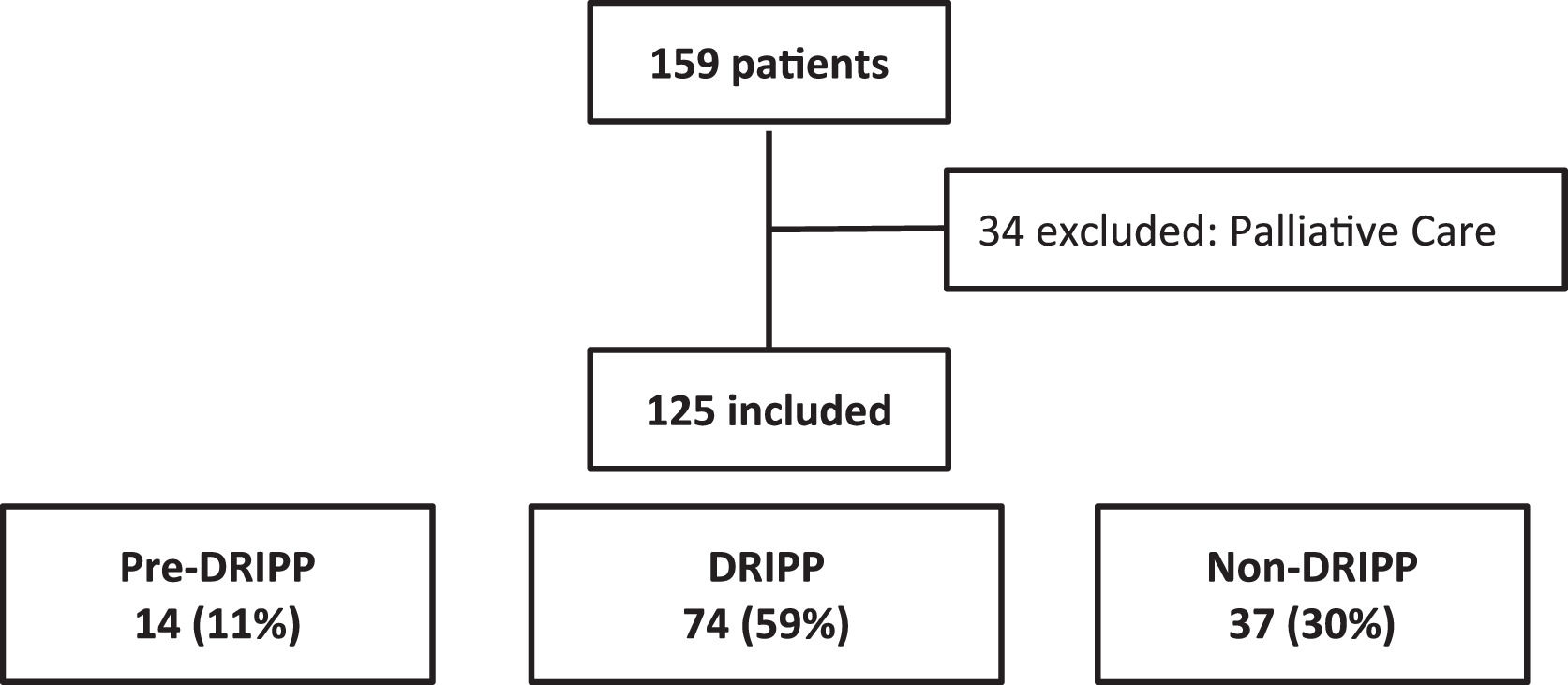
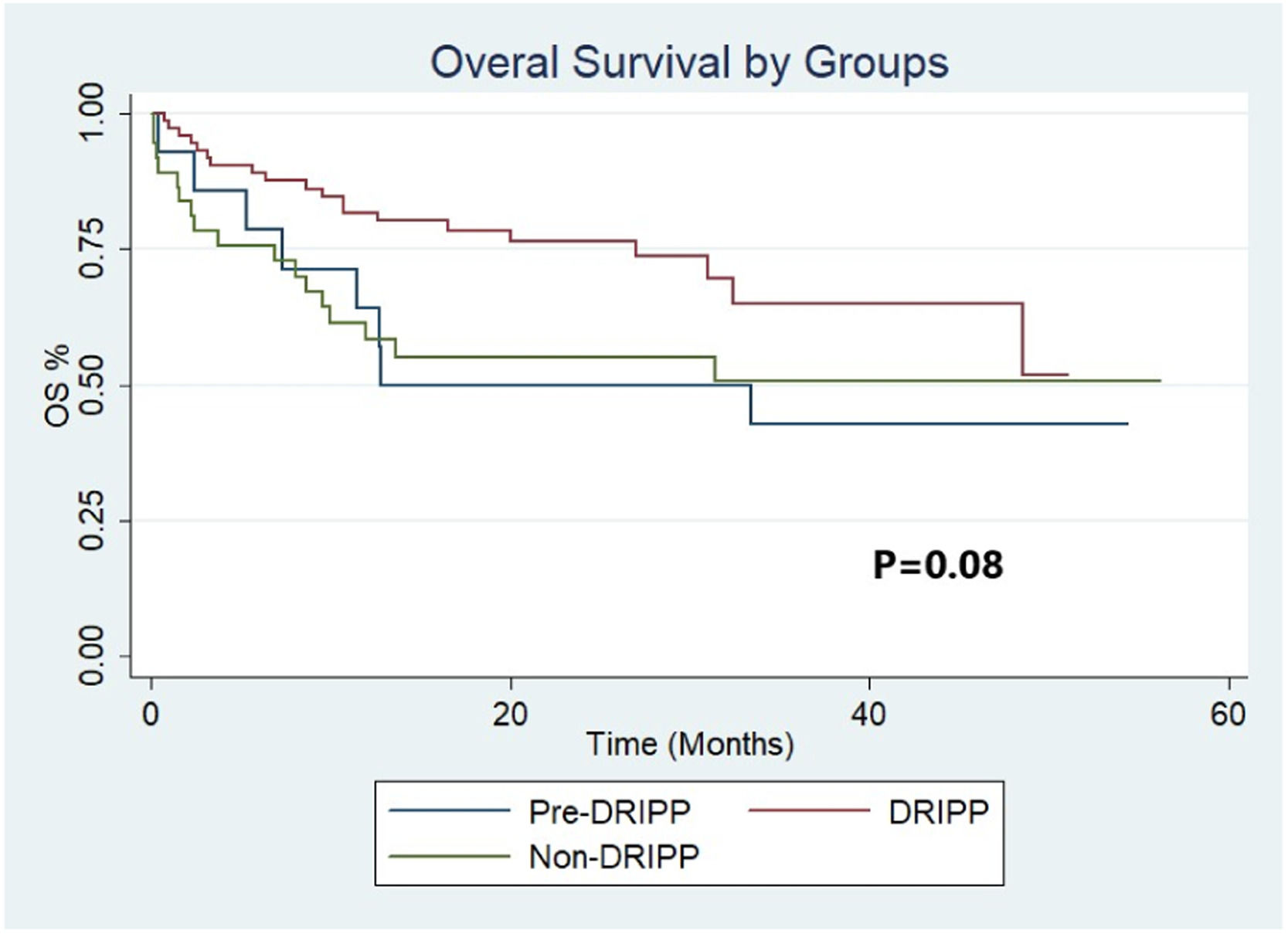
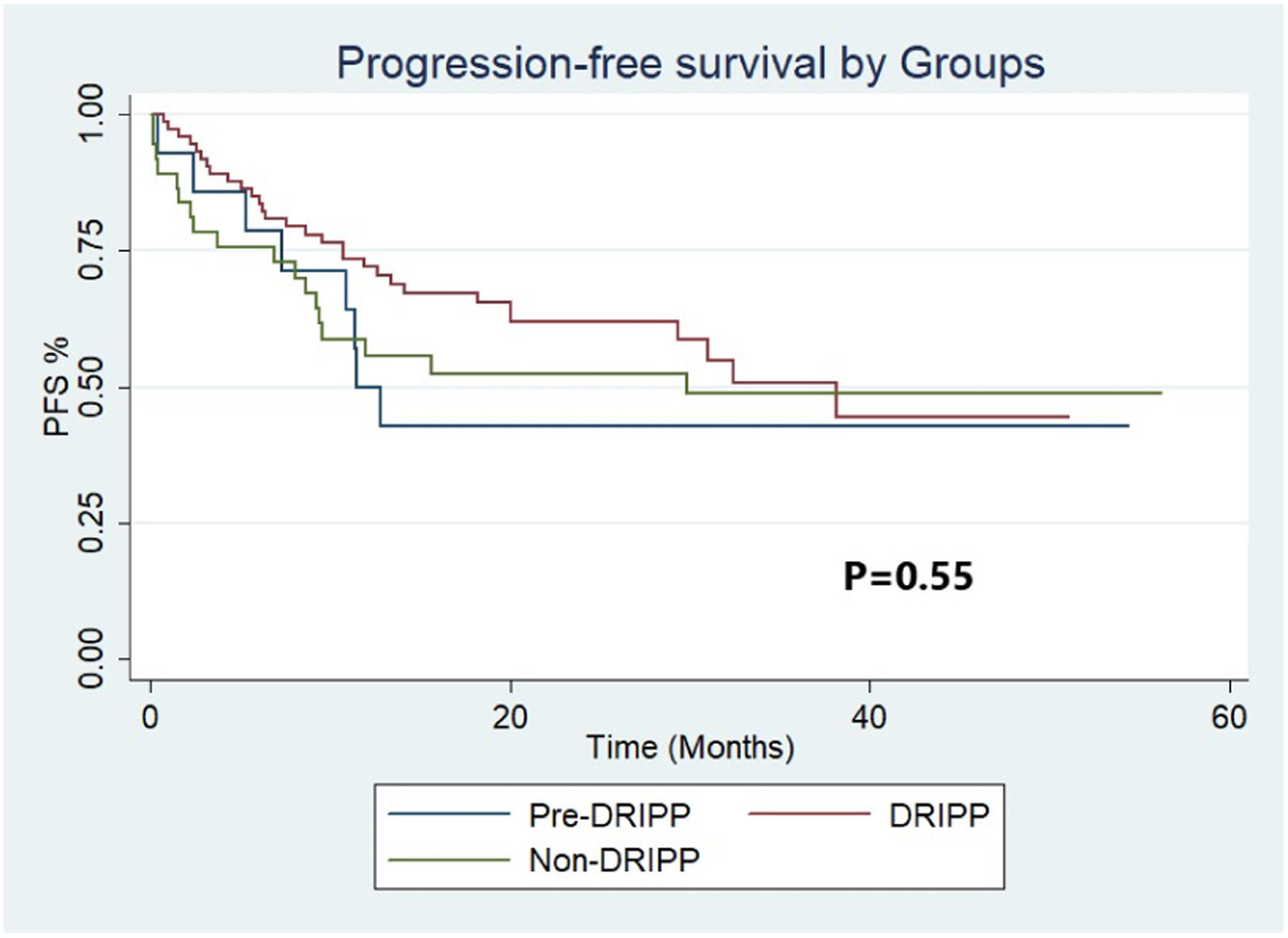
ResultsA total of 125 patients were analyzed. Fourteen (11%) patients in the pre-DRIPP group, 74 (59%) in the DRIPP group, and 37 (30%) in the non-DRIPP group. In 43 (58%) patients of the DRIPP group, some drug dose adjustments were made vs. 19 (15%) in the non-DRIPP (p = 0.03). There were no significant differences in terms of discontinuation of treatment or hematological toxicity between groups. The OS and PFS in one year was 64% (95%CI 34–83) and 50% (95%CI 23–72) for the pre-DRIPP group, 82% (95%CI 71–89) and 72% (95%CI 60–81) for the DRIPP group, 58% (95% CI 41–72) and 56% (95% CI 38–70) for the non-DRIPP group, (p = 0.08). The analysis was adjusted for probable confounders and no differences were found.
ConclusionsThis is the first study to evaluate the DRIPP as a decision-making tool in patients with lymphoma and showed a trend towards improvement in the OS in evaluated patients.
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma in adult patients and its annual incidence has been increasing since the 1970s. It is more frequent in the elderly population between 65 and 74 years.1,2 Given the aging of the world population, the diagnosis and treatment of DLBCL continues to be a relevant issue for health providers. The standard treatment is based on the combination of Rituximab with polychemotherapy, including anthracyclines. Because of Its comorbidities and toxicities, is not possible for a quarter of the patients to receive treatment.3,4 Some authors suggest that reducing the anthracycline dose would reduce the toxicity without having a negative impact on the overall survival (OS) of these patients.5
The Determination and Management of Risks for Practices and Procedures in the Elderly (DRIPP) is a multidimensional evaluation program in older adults who are in a cancer or surgical treatment plan created by the Hospital Italiano de Buenos Aires Geriatrics Service. It was initially designed for presurgical evaluation and was later extended to other clinical situations, such as chemotherapy in Oncology and Hemato-oncology.6 The objective is to qualify and quantify risks for a comprehensive patient stratification, while generating an individualized plan for clinical management. Geriatric variables (functional, cognitive and mood) included in the assessment are associated with complications, mortality, functional impairment and quality of life. The program has been shown to have an impact on decision-making.7–9
Since 2015, the Hospital Italiano de Buenos Aires (HIBA) has adopted the strategy of evaluating all patients over 65 years old planning to undergo chemotherapy treatment with the DRIPP. The main objective of this study was to evaluate the experience and effectiveness of this program. We calculated the OS, treatment-related mortality and progression-free survival (PFS) of patients who underwent the DRIPP evaluation versus those not evaluated. In addition, we estimated the intensity of the chemotherapy dose, discontinuation rate, toxicities and medical resources used for each subgroup of patients.
Materials and methodsDesign and scopeThis was a retrospective cohort study in elderly patients with a DLBCL diagnosis treated at the HIBA, Argentina.
PopulationPatients over 65 years old with a diagnosis of DLBCL according to the 2016 WHO criteria performed by a hematopathologist at the HIBA between January 2004 and December 2018 were included. We excluded patients without follow-up after diagnosis and those admitted to palliative care without further diagnostic evaluations. The systematic research was performed in the hospital's epidemiological sector using the Electronic Medical Record to identify patients. Moreover, the Geriatric The service database was reviewed with all the DRIPP evaluations of patients with hematological pathologies. Thus, 3 groups were defined: a historical cohort of patients with a diagnosis prior to the implementation of the DRIPP (pre-DRIPP group, January 2004–December 2014), a second cohort of patients with a diagnosis after January 2015 who were evaluated with the DRIPP (DRIPP group), and a group of patients who were diagnosed between January 2015 and December 2018, but were not evaluated with the DRIPP (non-DRIPP group). Rituximab was available to all patients, including the pre-DRIPP cohort.
InstrumentThe DRIPP is a multidimensional program whose objective is the detection, qualification, quantification and management of risks. To this end, it assesses the patient's frailty and functionality, classic comorbidities (historically assessed by the Charlson score) and new comorbidities (including dementia, mood disorders, impaired social network, polypharmacy and walk disorders).5 It consists of an evaluation performed by a geriatrician for an hour, where a detailed semi-structured interview with the patient is performed, as well as functional, cognitive and mood state systematized tests. Its objective is to provide the treating physician with a report on the real risk of the proposed treatment, help predict and minimize adverse effects, estimate the biological age and assess the patient's frailty. The information generated is taken into account by the treating team to make decisions related to whether or not to administer the chemotherapy and the possibility of adjusting schemes. The variables evaluated were collected from the prospective DRIPP registry of the Geriatrics service.
Statistical analysisDescriptive statistics were used for clinical and pathological characteristics. Quantitative variables are expressed as median and interquartile range and qualitative ones, as total number and percentage. The OS was assessed using the Kaplan-Meier method. For subgroup differences, a Log-Rank test was performed. A p-value less than 0.05 was considered statistically significant. The estimators are reported with their 95% confidence intervals. Statistical analysis was performed with Stata 14.
Ethical considerationsThis study was approved by the Hospital Research Protocols Ethics Committee (CEPI) and conducted in compliance with the Declaration of Helsinki.
ResultsA total of 159 patients were included in the study and 34 were excluded, due to the marked deterioration in their general condition. Thus, 125 patients were analyzed (Figure 1). The median age of the entire cohort was 74 (IQR 70–79) years and 63 (50.5%) were women. More than half of the patients had stage IV (n = 64, 51%) and the intermediate high and high International Prognostic Index (IPI) risk scores predominated (n = 58 of 100 evaluated, 58%).
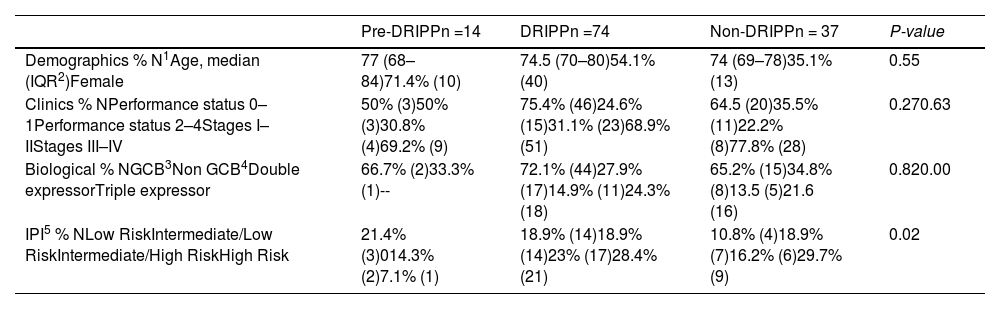
Fourteen (11%) patients were included in the pre-DRIPP group, 74 (59%), in the DRIPP group, and 37 (30%), in the non-DRIPP group. Patients from the pre-DRIPP era underwent some drug dose adjustment in only 3 cases (21%) vs. 43 (58%) in the DRIPP group and 16 (43%) in the non-DRIPP group (p = 0.03). The adjustments were predominantly at the start of the treatment in 49 (79%) patients, the main reason being frailty in 43 (70.5%), followed by comorbidities in 11 (18%) and treatment toxicity in 8 (12.9%). Among the patients receiving anthracyclines, 3 of 4 (75%) in the pre-DRIPP era had dose adjustments, compared to 37 of 51 (72%) in the DRIPP group and 11 of 19 (58%) in the non-DRIPP group. (p = 0.2). The average dose of anthracyclines was 146 mg/m2 in the DRIPP group vs. 168 mg/m2 in the non-DRIPP group, (p = 0.51) and the data was missing in the pre-DRIPP group (Table 1).
Baseline characteristics n = 125.
| Pre-DRIPPn =14 | DRIPPn =74 | Non-DRIPPn = 37 | P-value | |
|---|---|---|---|---|
| Demographics % N1Age, median (IQR2)Female | 77 (68–84)71.4% (10) | 74.5 (70–80)54.1% (40) | 74 (69–78)35.1% (13) | 0.55 |
| Clinics % NPerformance status 0–1Performance status 2–4Stages I–IIStages III–IV | 50% (3)50% (3)30.8% (4)69.2% (9) | 75.4% (46)24.6% (15)31.1% (23)68.9% (51) | 64.5 (20)35.5% (11)22.2% (8)77.8% (28) | 0.270.63 |
| Biological % NGCB3Non GCB4Double expressorTriple expressor | 66.7% (2)33.3% (1)-- | 72.1% (44)27.9% (17)14.9% (11)24.3% (18) | 65.2% (15)34.8% (8)13.5 (5)21.6 (16) | 0.820.00 |
| IPI5 % NLow RiskIntermediate/Low RiskIntermediate/High RiskHigh Risk | 21.4% (3)014.3% (2)7.1% (1) | 18.9% (14)18.9% (14)23% (17)28.4% (21) | 10.8% (4)18.9% (7)16.2% (6)29.7% (9) | 0.02 |
Fifty-five (74.3%) DRIPP evaluations were performed in non-hospitalized patients, while 19 (25.7%) were performed in hospitalized ones. The main results are described in Table 2. We must clarify that whether or not to perform the evaluation was the decision of the treating physician and the patient's health provider. Although the practice was recommended for all patients with lymphomas and candidates for an oncospecific treatment, there were physicians on the team who took longer to acquire the practice, or health providers who did not cover it, since it is not a universalized study outside our hospital. Unexpectedly, there were 30 patients (48% of 63 evaluated) whose DRIPP result concluded frailty, but chemotherapy was not contraindicated. Twenty-three (77%) of the frailty patients had to have adjusted chemotherapy vs. only 16 (51%) in the non-frailty patients, p = 0.038. The most frequent chemotherapy regimen was R mini CHOP in 12 and R CVP in 3 patients. In all cases, the DRIPP report included suggestions regarding modifications in the usual medication, geriatric recommendations for the patient and recommendations or an alarm guide for the treating physician.
DRIPP.
| Polypharmacy, median (IQR1)Charlson score, median (IQR)ADL1, median (IIC)Patient with nutritional risk, N % 3Frailty, N %Depression, N %Cognitive impairment, N % | 4 (IIC 0–7)3 (IIC 2–3)6 (IIC 5–6)26 (58%)*30 (48%)&#¿;&#¿;12 (18%)&#¿;&#¿;&#¿;17 (25%)&#¿;&#¿;&#¿; |
The treatment discontinuation rate was 20%, similar in the 3 groups. Hematological toxicities were also similar, while infectious complications predominated in the non-DRIPP group, with 19 (59.3%) patients, compared to the DRIPP group, with 21 (36%) and the pre-DRIPP, with 3 (27%) (p = 0.06). The number of non-scheduled consultations of the total cohort per patient was 2 (IIC 1-4), the number of unscheduled hospitalizations was 1 (IIC 0-1) and there were no differences between the groups (p = 0.29, p = 0.78).
The median follow-up of the full cohort was 20 months (IQR 9.4–33.2). The OS and PFS at 12 months were 64% (95%CI 34–83) and 50% (95%CI 23–72), respectively, for the pre-DRIPP group, 58% (95%CI 41–72) and 56% (95%CI 38–70) for the non-DRIPP group, and 82% (CI 95% 71–89) and 72% (CI 95% 60–81) for the DRIPP group (Figures 2 and 3). Although it did not reach statistical significance, this result showed a marked tendency favoring the DRIPP group in the OS (p = 0.08). The median OS and PFS of the full cohort were 38 and 61 months, respectively. There were 3 treatment-related deaths in the non-DRIPP group and none in the DRIPP group (p = 0.4). The analysis was adjusted for probable confounders and no differences were found in the distribution of the variables stage, IPI, performance status, cell of origin, or double or triple expressors. Finally, outcomes were worse in patients with frailty, with a median OS of 32 months, while it was not reached in the non-frail patients (p = 0.00).
DiscussionThis study describes the characteristics and evolution of a cohort of patients over 65 years old diagnosed with DLBCL, with a special focus on the DRIPP geriatric assessment program implemented at the beginning of 2015. The geriatric assessment tools help to detect clinical characteristics of patients and, therefore, the possibility of performing individualized oncological treatments. Unfit patients have a lower OS and greater treatment-related toxicity and this cheap and easily reproducible clinical tool may be the strategy to objectively identify at-risk patients who could benefit most from reduced chemotherapy.10–12
In this study, a trend towards an increased OS was observed in patients evaluated with the DRIPP vs. those not evaluated (OS at 12 months 82% vs. 64%); it is likely that a part of this difference is due to the impact of the evaluation recommendations and their ability to identify which patients benefit from adjusting chemotherapy doses. Although the study requires further follow-up over time to assess the OS, the initial results are encouraging. Consistent with these findings, in a paper from the Italian Lymphoma Oncohaematology Group published by M. Spina et al., a comprehensive geriatric assessment was used to classify 100 patients > 65 years of age with DLBCL into “fit”, “unfit” and “frail”. They received treatment with immunochemotherapy with adjusted doses based on this classification, obtaining very good PFS and OS results for the entire cohort (60% and 80% at 5 years, respectively).13 In a previous study by A. Tucci et al., which prospectively evaluated 84 patients > 65 years of age with DLBCL, a comprehensive geriatric evaluation allowed for the detection of lower-risk patients who effectively benefited from the immunochemotherapy treatment. Patients identified as frail had a markedly lower OS, regardless of whether they received treatment with curative or palliative intent (median OS not achieved vs. 8 months, p < 0.001).14
Even though there was a special interest in evaluating differences in the toxicity and in the use of resources associated with the health system (non-scheduled consultations and hospitalizations), the present study could not demonstrate an impact on these objectives. This may be due to the small sample size of patients in the pre-DRIPP group.
We suggest the use of the DRIPP over other geriatric tools (such as the widely used “comprehensive geriatric assessment” [CGA]), since it combines multiple features that allow for a comprehensive and standardized approach.14,15 The CGA incorporates the evaluation of different aspects of older adult health and primarily serves diagnostic and treatment recommendation plan purposes. The DRIPP is a program that includes the CGA, but is not limited to it, as it implements action plans, such as nutritional optimization, social intervention, medication reconciliation and changes, management of risk factors for confusion syndrome and optimization of analgesic treatment, with the possibility of patient follow-up and weekly team meetings for patient discussions. The DRIPP has also an assessment of multiple geriatric dimensions, validated scales, short study time (40–60 minutes), short training period for geriatricians, clinicians, or even nurses, and the correct application of the concepts of frailty, functionality and burden of disease. In addition, we have evidence that shows good performance of the tool in the prediction of adverse events and mortality in oncohematological disease.7–9 In the future, it will be interesting to evaluate the correlation between the DRIPP, the CGA and the evolution of patients.
This study has inherent weaknesses in its design. As it assesses a retrospective cohort, the data collection of some clinical variables could be inaccurate or incomplete. In any case, the integrated system of the Electronic Medical Record allows for the acquisition of high-quality administrative information, giving reliability to our findings. The differences in the size of the samples, with few patients included in the pre-DRIPP group, make it difficult to compare these groups. The low number of patients prior to 2014 is due to the fact that most of their medical records were not digitized (especially in the period prior to 2010). In addition, in recent years there has been a greater tendency to perform a complete evaluation and start an oncospecific treatment, even in elderly adult patients, probably encouraged by the published results with adjusted schemes in this age group. The fact that the patients have performed the DRIPP evaluation or not in the last follow-up period may also represent a bias. This decision was not randomized, but depended on the indication of the attending physician and the health insurance provider. However, the most important variables were adjusted for the main confounders (stage, IPI, performance status, cell of origin and double or triple expressors) without finding any modifications.
On the other hand, this is the first study to evaluate the DRIPP as a decision-making tool in patients with lymphoma and it could be the starting point to give continuity to a program that aims to maximize the effectiveness of treatments in older patients, avoiding overtreatment and decreasing toxicity. Another strength is that the patients were recruited with a double systematic search by the Epidemiological and the Geriatrics Sectors, minimizing the possibility of not including patients within the study period, especially after 2015. On the other hand, the cohort of patients evaluated with the DRIPP included a significant number of patients, which makes the data in this subgroup evaluated with the DRIPP more robust. Finally, the evaluation data was taken from the DRIPP registry of the hospital's Geriatrics Service, which was prospectively loaded from the beginning of the program, giving greater quality and making the collected data more complete.
ConclusionWe believe that the DRIPP is not just a geriatric evaluation to define the frailty of the patient. The geriatrician helps the hematologist in charge to modify or prescribe medication, gives recommendations for daily life during the chemotherapy period and participates in the decision-making by the medical team in charge of decisions related to the type and intensity of the chemotherapy. Our study shows that a comprehensive geriatric evaluation program in older adult patients is positive and promising and favors the continuation of a multidisciplinary approach in the future. The experience has been encouraging, so we will continue working together with the Geriatrics Sector to improve the tool, optimize its use, define customized therapeutic strategies based on its results and subsequently evaluate them prospectively.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None.