Diversity in Classical Hematology Research
More infoPost-transplant lymphoproliferative disorders (PTLDs) are a heterogeneous group of lymphoid proliferations occurring after solid organ or bone marrow transplantation. The primary aims of our study were to characterize cumulative incidence of PTLDs, clinical and pathological features according to the Epstein-Barr virus (EBV) status and survival.
MethodsThis was a retrospective cohort study on adult and pediatric patients, from January 2001 to December 2017. The cumulative incidence of PTLD was calculated by analyzing all the patients transplanted at our hospital, based on the database of the Organ Donation and Ablation Authority of Argentina (INCUCAI). The Kaplan-Meier method was used to plot the survival.
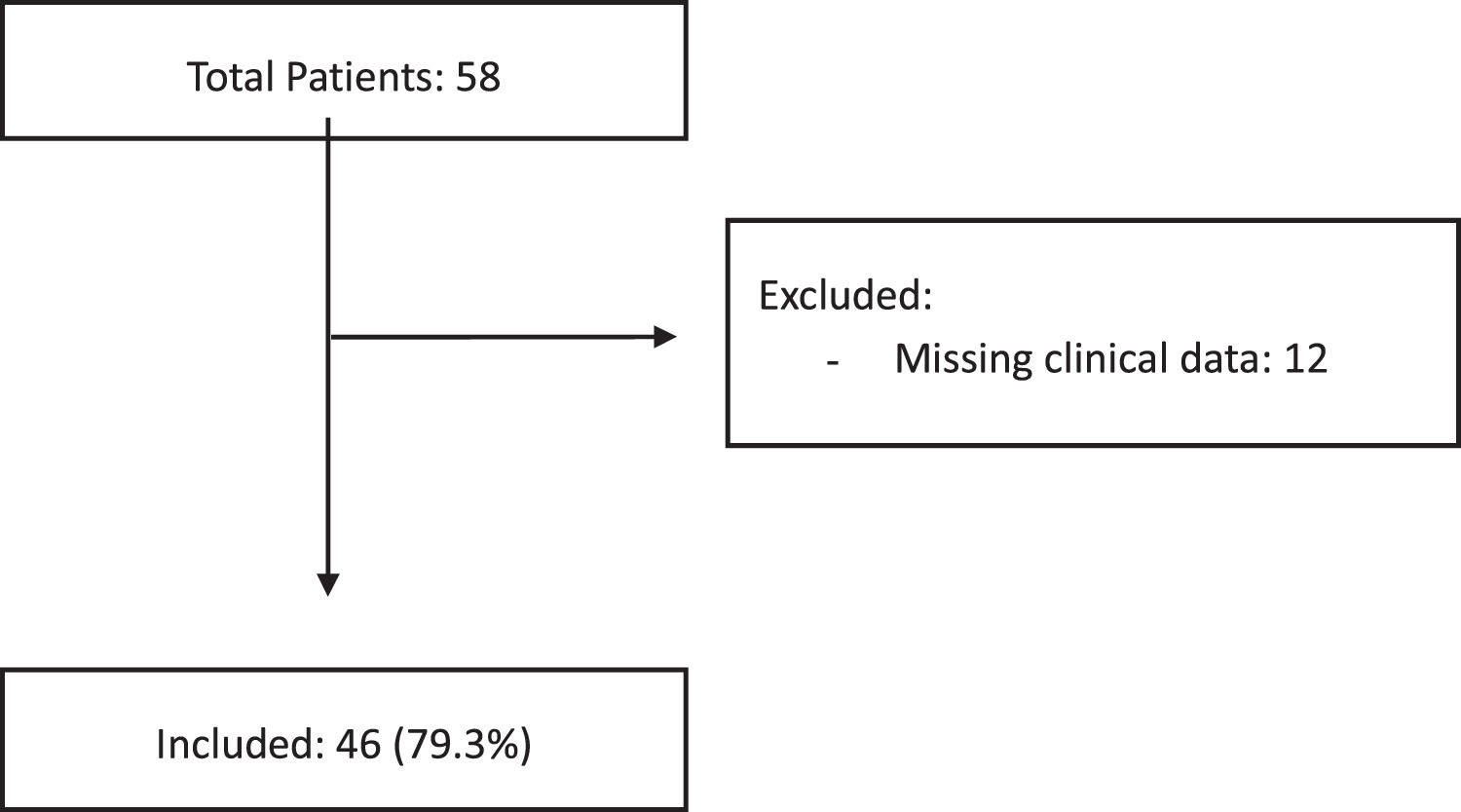
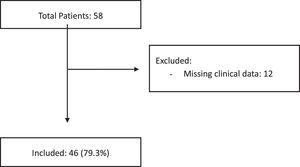
ResultsFifty-eight cases of biopsy-confirmed PTLD were identified and 12 cases of clinical data were incomplete and these patients were excluded. The median age at the time of the PTLD diagnosis was 17.5 years (interquartile range [IQR] 9 - 57). The median interval between transplant and PTLD diagnosis was 39 months (IQR 9 - 113). The most commonly transplanted organ was the liver (24 cases, 52.2%), followed by kidney (20 cases, 43.5%). The Epstein-Barr encoding region in situ hybridization (EBER ISH) was positive in 29 (69.8%) of the 43 evaluable biopsies. The PTLD cumulative incidence was 1.84% (95%CI 1.77 - 1.91) for solid organ and 0.84% (95%CI 0.48 - 1.2) for bone marrow transplant patients. The overall survival rate at 5 years was 0.77 (95%CI 0.61 - 0.87). Subgroups by the EBV EBER status, transplant type, PTLD subtype and age group (adult vs. pediatric) showed no statistically significant association with the overall survival.
ConclusionThe PTLD incidence was similar to that of previous series and the EBER did not appear as a relevant factor in our patient survival.
Post-transplant Lymphoproliferative Disorders (PTLDs) are a set of rare, but potentially severe complications, in solid organ (SOT) and bone marrow transplant (BMT) recipients. The PTLDs comprise a heterogenous morphological spectrum, ranging from benign polyclonal hyperplasia to aggressive lymphomas. In 2017, the World Health Organization (WHO) reclassified PTLD categories into four main groups (non-destructive PTLDs, polymorphic PTLDs, monomorphic PTLDs and classic Hodgkin lymphoma PTLDs).1 Most PTLDs are B-cell neoplasms (with diffuse large B-cell lymphomas [DLBCLs], representing over 80% of the cases), and only 5 to 10% are T cell/NK cell types or Hodgkin lymphoma type.2
The Epstein-Barr virus (EBV) plays a significant role in the pathogenesis of PTLDs (up to 70% of the cases are EBV positive), associated with chronic immunosuppression and T cell inhibition, however, about 30% of the patients are EBV negative.1,3
The PTLD incidence varies depending on immunosuppressive regimens, the type of transplanted organ (1 - 2% kidney, liver and bone marrow; 5 - 10% heart, lung or bowel) and age.4,5
Several series of patients with PTLD have been published, all of which present limitations due to heterogeneity in the patient population, incomplete data on the histopathological subtype and the low frequency of this entity. In our country, available data on this entity is even more limited.2,6-9
The primary aims of our study were to characterize clinical and pathological features of PTLDs, survival (global and by histological subgroups), cumulative incidence and clinical outcome according to the EBV status.
Materials and methodsDesign and scopeThis was a retrospective cohort study of adult and pediatric patients diagnosed with PTLD, which was confirmed by histological assessment, at the Hospital Italiano de Buenos Aires, Argentina, between January 2001 and December 2017.
PopulationPatients were identified from the hospital databases, electronic medical records and Pathology Department electronic files. All specimens were reviewed by two expert pathologists (DK, HGR) and classified according to the 2017 WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues as non-destructive PTLDs, polymorphic PTLDs, monomorphic PTLDs and classic Hodgkin lymphoma (CHL) PTLDs.1
Variables
The following demographic and clinical variables were considered in the statistical analysis: age, gender, affected organ, nodal and extranodal involvement, transplanted organ, International Prognostic Index (IPI) at diagnosis, stage at diagnosis, transplant type, B-symptoms, date of transplant, antiviral prophylaxis, use of rabbit anti-thymocyte globulin (rATG) and other pre-transplant therapy, immunosuppressors, treatment, treatment response, graft organ involvement, development of more than one PTLD after transplantation, time to PTLD development, viral serology, death, date of death, cause of death and date of the last patient contact. Patients were treated by physicians of the Divisions of (Adult) Hematology and Pediatric Hematology-Oncology.
Histopathological tests: The histopathological diagnosis was performed using routine histological techniques in formalin-fixed paraffin-embedded (FFPE) biopsies and automated immunohistochemistry (Ventana Benchmark XT, Roche). The following markers were used: CD20, CD3, CD30, CD10, Bcl2, Bcl6, IRF4/MUM1, CD138, CD79a, CMYC and Ki67.
In addition, in situ hybridization (ISH) for Epstein-Barr virus (EBV)-encoded RNA (EBER) was performed using the EBER 1 DNP probe (Ventana, Roche) and the ISH iView Blue Plus detection kit (Ventana, Roche), designed to bind to EBV non-coding RNAs (Epstein-Barr Virus-Encoded RNA 1, EBER 1). Cases were considered positive if they presented intense nuclear staining in most of the lymphoid cells.
Statistical analysisDescriptive statistics was used for the clinical and pathological features. Quantitative variables were expressed as median and interquartile range, whereas qualitative variables, as total number and percentage. Variables with missing data are summarized in Table 1. The T test was used to assess time differences (in months) to PTLD onset/development in transplanted patients (liver vs. kidney, adult vs. pediatric).
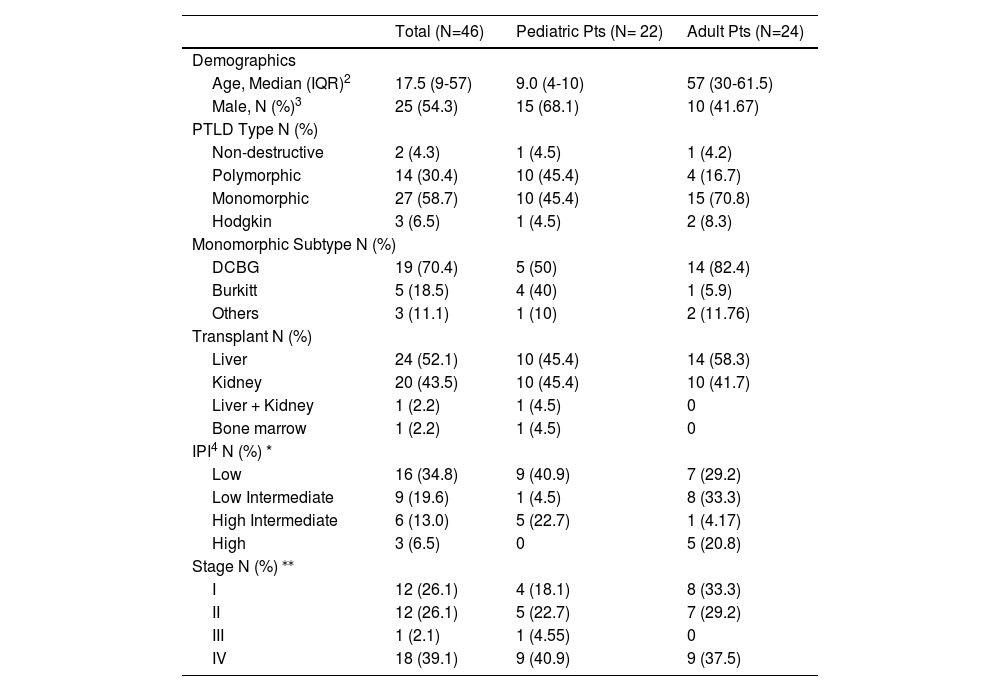
Clinical characteristics of adult vs. pediatric patients with PTLD.1
| Total (N=46) | Pediatric Pts (N= 22) | Adult Pts (N=24) | |
|---|---|---|---|
| Demographics | |||
| Age, Median (IQR)2 | 17.5 (9-57) | 9.0 (4-10) | 57 (30-61.5) |
| Male, N (%)3 | 25 (54.3) | 15 (68.1) | 10 (41.67) |
| PTLD Type N (%) | |||
| Non-destructive | 2 (4.3) | 1 (4.5) | 1 (4.2) |
| Polymorphic | 14 (30.4) | 10 (45.4) | 4 (16.7) |
| Monomorphic | 27 (58.7) | 10 (45.4) | 15 (70.8) |
| Hodgkin | 3 (6.5) | 1 (4.5) | 2 (8.3) |
| Monomorphic Subtype N (%) | |||
| DCBG | 19 (70.4) | 5 (50) | 14 (82.4) |
| Burkitt | 5 (18.5) | 4 (40) | 1 (5.9) |
| Others | 3 (11.1) | 1 (10) | 2 (11.76) |
| Transplant N (%) | |||
| Liver | 24 (52.1) | 10 (45.4) | 14 (58.3) |
| Kidney | 20 (43.5) | 10 (45.4) | 10 (41.7) |
| Liver + Kidney | 1 (2.2) | 1 (4.5) | 0 |
| Bone marrow | 1 (2.2) | 1 (4.5) | 0 |
| IPI4 N (%) * | |||
| Low | 16 (34.8) | 9 (40.9) | 7 (29.2) |
| Low Intermediate | 9 (19.6) | 1 (4.5) | 8 (33.3) |
| High Intermediate | 6 (13.0) | 5 (22.7) | 1 (4.17) |
| High | 3 (6.5) | 0 | 5 (20.8) |
| Stage N (%) ⁎⁎ | |||
| I | 12 (26.1) | 4 (18.1) | 8 (33.3) |
| II | 12 (26.1) | 5 (22.7) | 7 (29.2) |
| III | 1 (2.1) | 1 (4.55) | 0 |
| IV | 18 (39.1) | 9 (40.9) | 9 (37.5) |
The cumulative incidence of PTLD was calculated by analyzing all the patients transplanted at our hospital, based on the database of the Organ Donation and Ablation Authority of Argentina (INCUCAI), the national agency promoting, standardizing, coordinating and supervising donation and transplantation of organs, tissues and cells in Argentina.10,11 This analysis included patients with biopsy-confirmed PTLD and excluded those with incomplete clinical data.
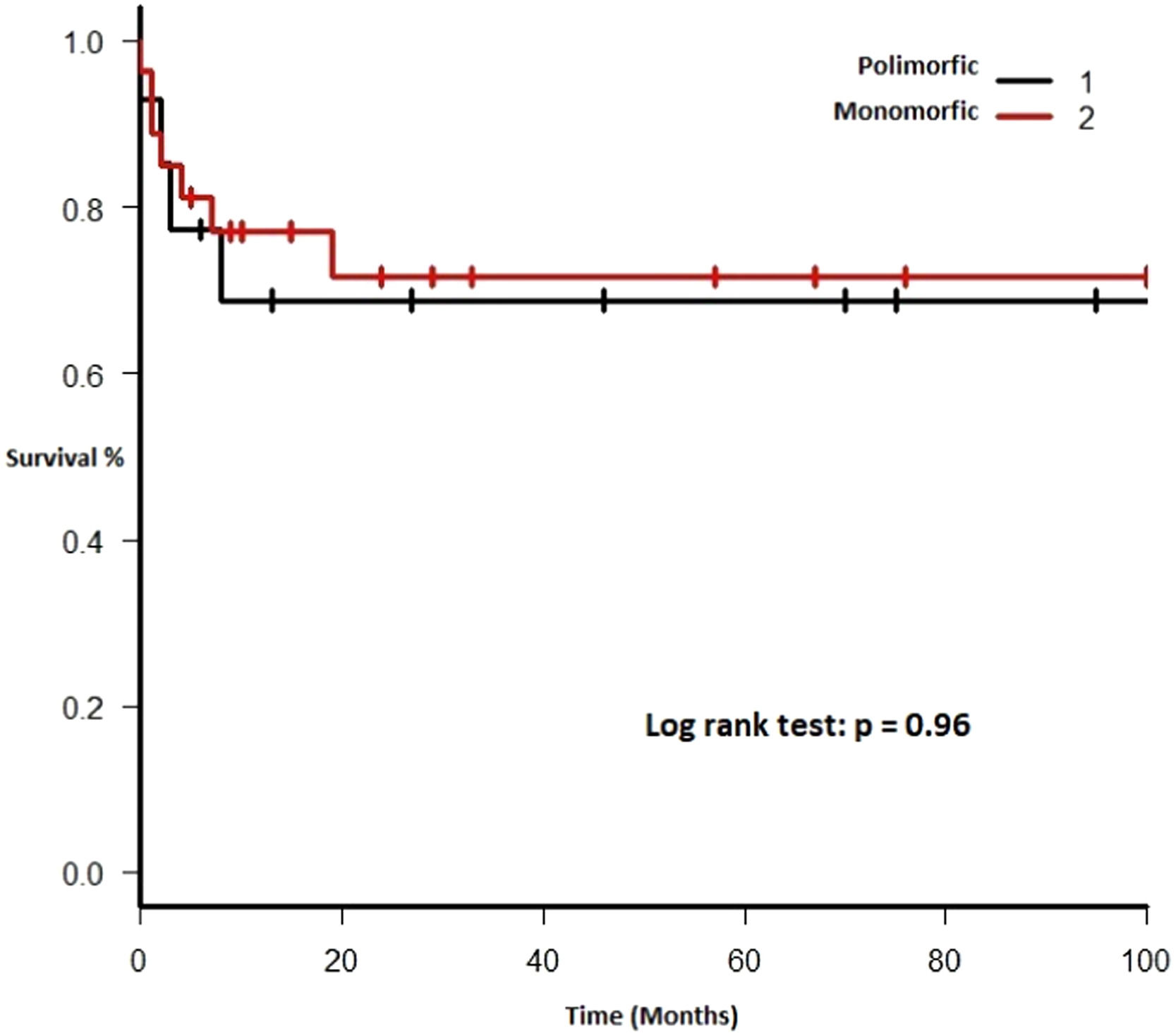
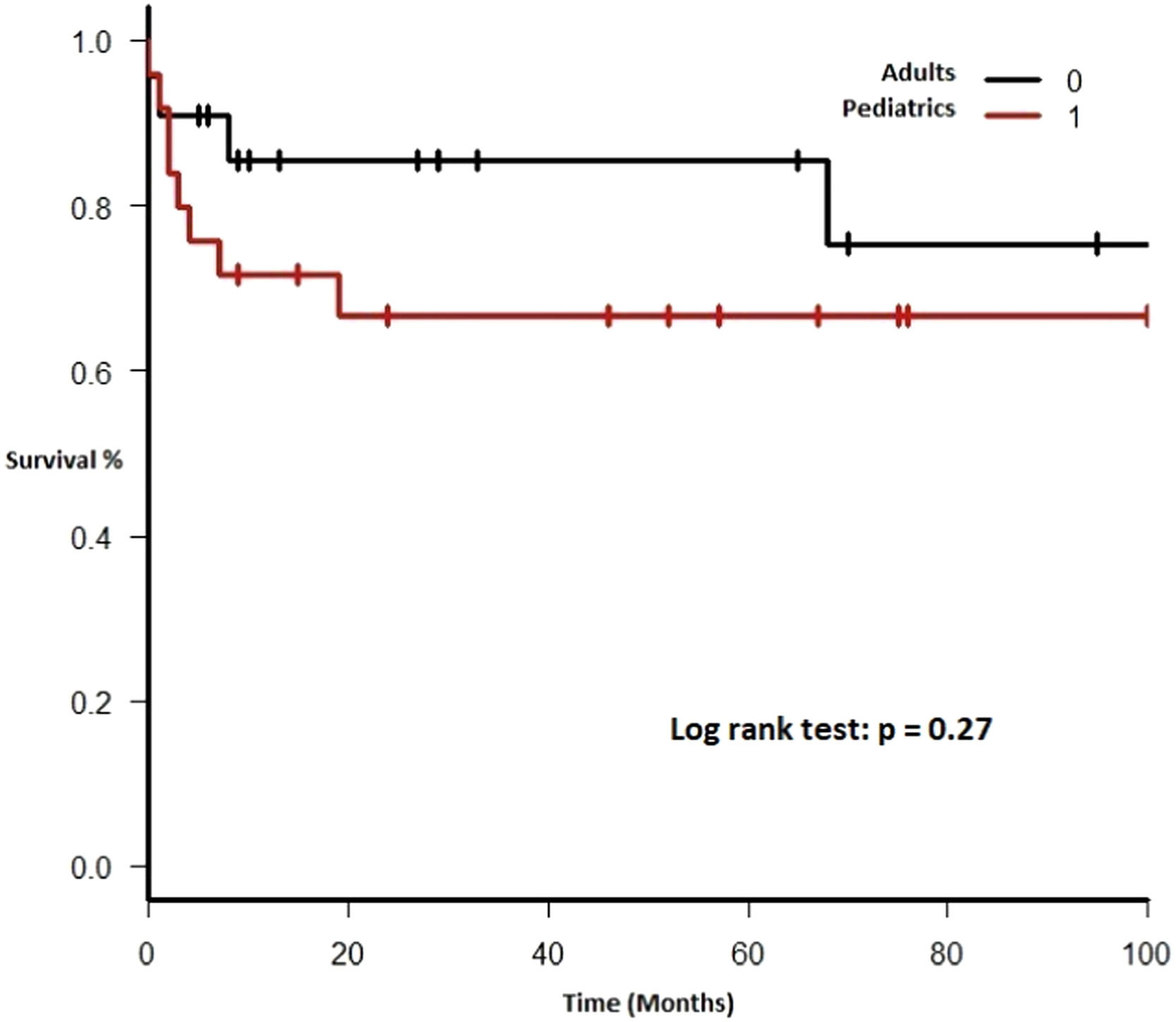
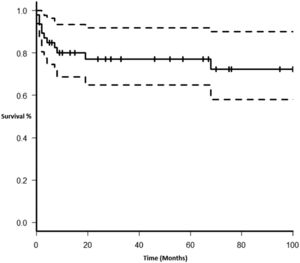
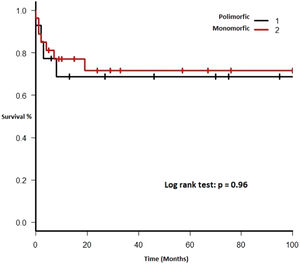
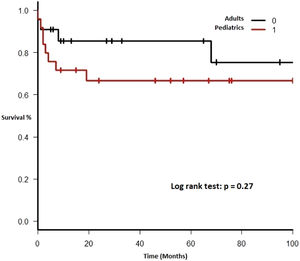
Each included patient was retrospectively analyzed from the date of diagnosis until death, loss to follow-up or study end (December 31, 2017). The Kaplan-Meier method was used to plot survival (in months). Subgroups were analyzed comparing EBV EBER status (positive vs. negative), PTLD subtype (polymorphic vs. monomorphic), transplant type (liver vs. kidney), and age group (adult vs. pediatric). The log-rank test was used to assess statistical significance. Data were analyzed using the Strata Statistical Software, version 14.0 (Figure 1).
Ethical considerationsThis study was approved by the hospital's Research Protocols Ethics Committee (CEPI), conducted in compliance with the Declaration of Helsinki and reported according to the STROBE Guidelines.
ResultsBaseline characteristicsBetween January 2001 and December 2017, 3,222 transplants were performed at the Hospital Italiano de Buenos Aires. These included 3,103 solid-organ and 119 bone marrow transplants.10
Patient characteristicsFifty-eight cases of biopsy-confirmed PTLD were identified. As a national referral center, there are patients who only undergo the transplant and receive the diagnosis of PTLD at our hospital, but the management of the pathology is carried out in their city of origin. In 12 cases, the clinical data were incomplete, and these patients were excluded from the study. Most PTLDs developed in solid-organ transplant recipients (45 patients) and only one, in a bone marrow transplant recipient. The median age at the time of the PTLD diagnosis was 17.5 years (interquartile range [IQR] 9 - 57). There was a slight male predominance (25 cases; 54.3%). One patient was positive for hepatitis B (2.2%). No patient was hepatitis C or HIV positive. The EBV was positive in 13 of the 27 patients analyzed (48.1%). The cytomegalovirus (CMV) was positive in 5 (17.2%) of the 29 patients analyzed.
Transplant-related characteristicsThe most commonly transplanted organ was the liver (24 cases; 52.2%), followed by kidney (20 cases; 43.5%), one case of liver-kidney transplantation (2.2%) and one case of unrelated allogenic bone marrow transplant (2.2%).
The PTLD cumulative incidence was 1.84% (95%CI 1.77 - 1.91) for solid organ transplantation and 0.84% (95%CI 0.48 - 1.2) for bone marrow.
The median interval between transplant and PTLD diagnosis was 39 months (IQR 9 - 113) for the complete cohort, 48 months (IQR 13.5 - 114.5) for kidney transplants and 40 months (IQR 8 - 100) for liver transplants (p = 0.9); 62 months (IQR 13 - 168) for adults and 32 months (IQR 8 - 61) for pediatric patients (p = 0.07).
Seven patients (16.7%) developed more than one PTLD, while there was graft organ involvement in 11 (23.9%) patients.
At the time of the PTLD diagnosis, most patients (n = 45; 97.8%) were on immunosuppressive maintenance therapy, with the predominance of tacrolimus (n = 25; 55.5%) and mycophenolate (n = 21; 46.7%).
The pre-transplant conditioning data was obtained for 26 patients; most of them (n = 17; 65.4%) received some kind of conditioning therapy, being rabbit anti-thymocyte globulin (rATG) (n = 7; 30%), basiliximab (n = 1; 3.8%) and daclizumab (n = 1; 3.8%) the most frequent regimens.
Disease-related characteristicsHistological assessment showed that most of the cases (n = 27; 58.7%) were monomorphic PTLDs and 14 cases (30.4%) were polymorphic PTLDs. The most frequent monomorphic PTLD subtypes were DLBCLs (n = 19; 70.4%) and Burkitt lymphomas (n = 5; 18.5%). See Table 1 for further details. Extranodal disease was present in most patients (n = 32; 69.6%). The EBER ISH was positive in 29 (69.8%) of the 43 evaluable biopsies. Eighteen (42.9%) patients presented with stage IV disease at diagnosis. The IPI score was “low risk” or “low-intermediate risk” in 25 (71.4%) cases.
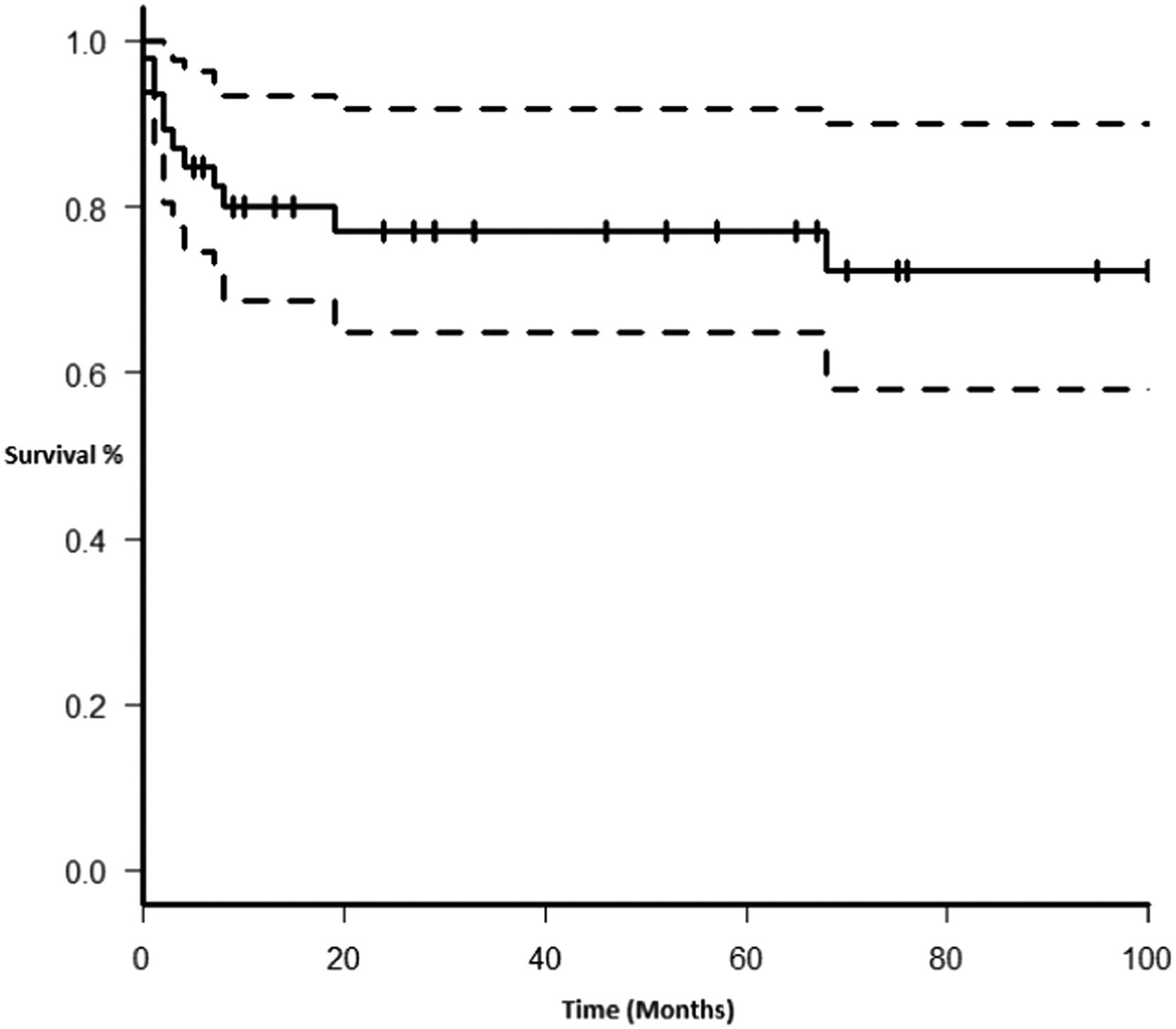
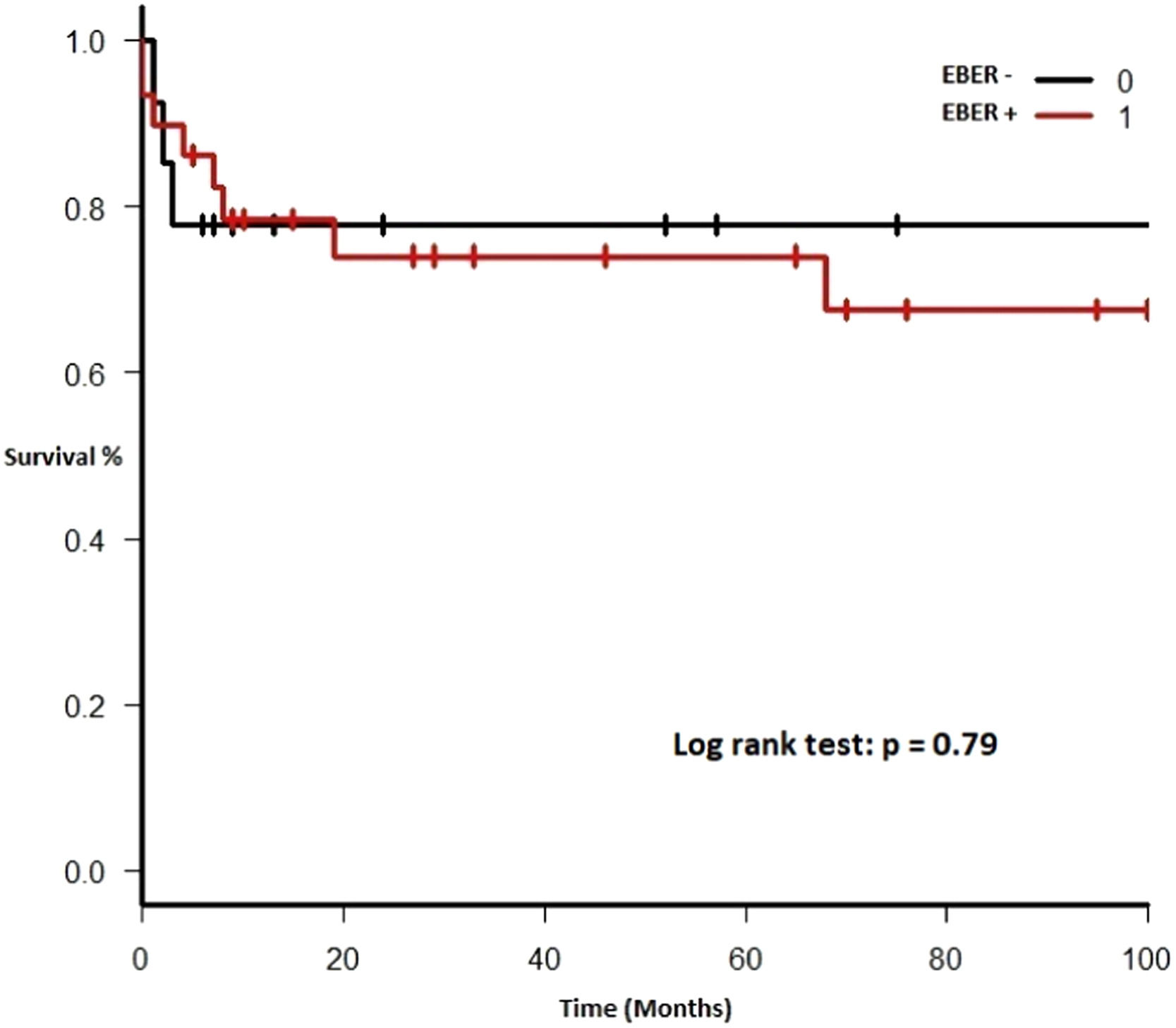
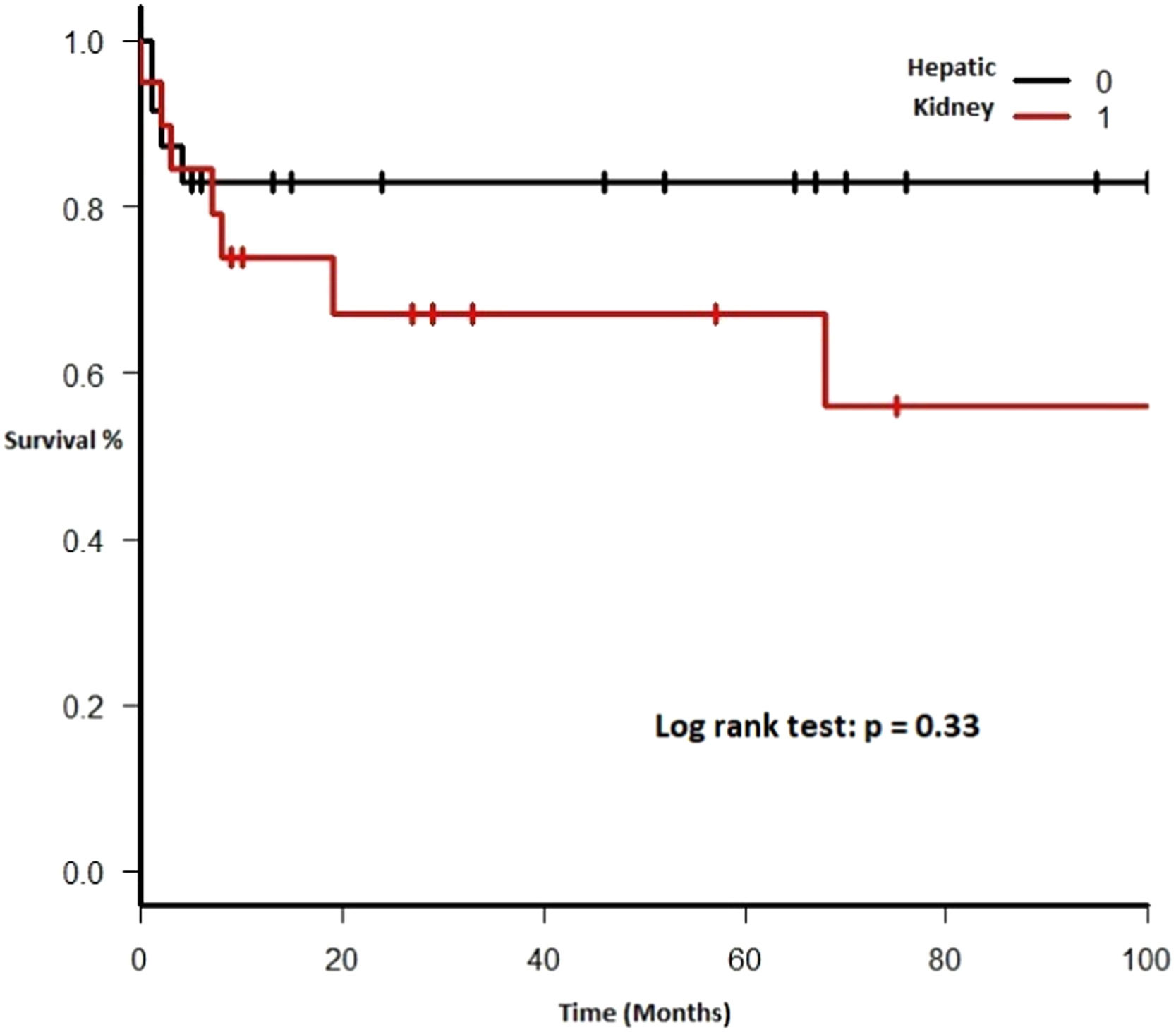
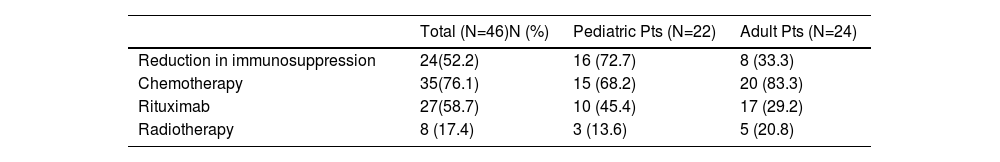
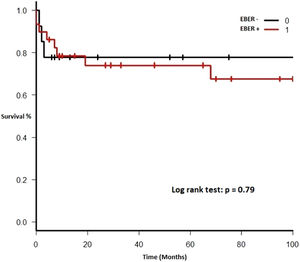
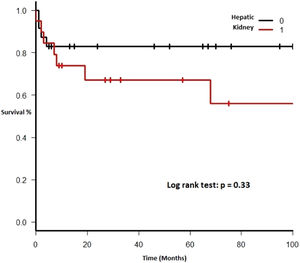
Therapy and outcomeAfter diagnosis of the PTLD, 44 (95.6%) patients received treatment, 35 (76.1%) received chemotherapy, 27 (58.7%), rituximab and 24 (52.2%) were treated with a reduction in immunosuppression (RIS) (Table 2). The median of relapse-free survival was 49 months (11.5 - 97.5). Twelve (26.1%) patients died, 8 (66.7%) from causes related to lymphoma. The median of time to death was 2 months (IQR 1 - 8). Figures 2-5 show the overall survival (OS) and subgroups survival curves. Overall survival rate at 5 years for the whole cohort was 0.77 (95%CI 0.61 - 0.87) (Figure 2). Subgroups by the EBV EBER status, transplant type, PTLD subtype and age group (adult vs. pediatric) showed no statistically significant association with the overall survival (Figures 3-6).
Treatment in patients with PTLD.1
The PTLDs comprise a spectrum of lymphoid proliferations, ranging from polyclonal hyperplasia to extremely aggressive lymphomas. Our study describes a cohort of 46 patients with PTLD from a 20-year retrospective sweep in the database of a University Hospital in Buenos Aires.
Monomorphic PTLDs (58.7%) were the subtype most frequently observed, with DLBCL being the most common histology (19 cases; 41.3%).
Other subtypes included Burkitt lymphoma (n = 5; 10.8%), plasmablastic lymphoma (n = 1; 2.1%), cutaneous MALT lymphoma (n = 1; 2.1%) and peripheral T-cell lymphoma CD4+ NOS (n = 1; 2.1%). Thirty-two (69.6%) patients presented extranodal disease. Classical Hodgkin lymphoma-type PTLD was observed in 3 cases (6.5%). Polymorphic PTLD represented 14 cases (30.4 %). Non-destructive early lesions were observed in 2 cases (4.3%).
The spread of histological subtypes is in agreement with data from other series. Yoon et. al. reported a higher incidence of non-destructive lesions (14%), while the distribution of polymorphic (30.2%) and monomorphic (51.2%) subtypes was similar.2 Similar to other series, the EBV+ PTLD was approximately 70%.12-14
The PTLD cumulative incidence was 1.84% (95%CI 1.77 - 1.91) for solid organ transplantation and 0.84% (95%CI 0.48 - 1.2) for bone marrow. The incidence of kidney and liver transplant recipients was slightly higher than that of other series. Yoon et. al. reported an incidence of 0.78% and 0.77%, respectively. Patients in this study were mostly liver and kidney transplant recipients, although there were also 9% of heart and 14% of bone marrow transplants. The median interval between the transplant and PTLD diagnosis was 44.5 months, similar to our study.2
The study by Francis A. et. al., using the Australian and New Zealand Dialysis and Transplant Registry, showed a PTLD incidence of 2% for kidney transplant recipients. This increased incidence might be related to the extended follow-up of patients (for more than 50 years) and the better prognosis for kidney transplant recipients, when compared to other transplanted organs.15
The experience in Argentina is more limited. The incidence in our study is consistent with that of Mendizabal M. et. al., which included liver transplant recipients at various centers in South America. The cumulative incidence in this study was 1.7%, with the mean time of 39.7 (SD ± 35.2) months from the transplant to PTLD diagnosis.8 Fernández MC et. al. published a 13-year experience in a population of pediatric liver recipients. The incidence of PTLD was 5.7%, with an average time from the transplantation to PTLD diagnosis of 25 months (range 7 - 47 months).9
The literature data on the PTLD incidence in bone marrow transplant recipients is more heterogenous. In a retrospective series of more than four thousand allogeneic hematopoietic stem cell transplants, Styczynski J et. al. reported an incidence of PTLD of 3.22%, ranging from 1.2% for the matched-family donor and 11.24% for mismatched unrelated donor recipients. [7] On the other hand, Yoon et. al. published a series of 747 bone marrow transplant recipients with a PTLD incidence of 0.8%. Both studies, as well as ours, showed a high percentage of PTLD development within one year of diagnosis.2 However, our series included only one bone marrow transplant patient with PTLD, therefore it is difficult to compare populations with the findings of these authors.
Of note, our cohort lacks post-heart, lung and bowel transplant PTLD cases, the three subgroups with the highest incidence reported in the literature.4,5 This phenomenon might in part be explained by the poor prognosis for lung and bowel transplant patients in our country and the low indication for transplantation in these patients.
The current standard of care is reduction in immunosuppression as the first-line treatment, which might lead to cure in non-invasive and polymorphic PTLDs.16-18 For the remaining PTLD subtypes, there was only one clinical trial showing good results after sequential treatment with rituximab followed by chemotherapy.17 Reduction in immunosuppression was only seen in 50% of the patients. This may be due to the fact that part of the PTLDs had been diagnosed before the application of the current treatments and standards of care and that the immunosuppressive regimen is administered by the physician indicating transplantation, rather than by hematologists, in our country. Fortunately, reduction in immunosuppression has become widely accepted in recent years.
Survival rates in our analysis were consistent with those of international series. Bishnoi et. al. published a cohort of 141 patients with an overall survival of 15 years, with a median of 5 and 1.32 years in adult and elderly patients, respectively.19 Trape R et. al. reported a survival of 69% at 3 years and 65% at 5 years; however, disease-free survival was 77 months, remarkably higher than ours.17 Styczynski J. et. al. observed a 12-month survival of 69.2% (CI 61 - 76) in bone marrow transplant recipients.7 In our study, survival at 5 years was 46.2%, strikingly higher than that of Mendizabal M. et. al., which may be due to the fact we included all PTLD subtypes, with a high share of kidney transplant patients, who have a better overall prognosis.8 Finally, overall survival was not different in the subtype of EBER+ PTLD patients. Although our hypothesis is exploratory and the result was negative, the number of patients in our cohort is low and further studies including more patients are needed to confirm this finding.
This is the largest cohort of patients in Argentina and the first to determine the incidence of PTLDs in solid organ and bone marrow transplants, making available local data for better understanding our patient characteristics and prognosis. Moreover, it contributes valuable information in terms of the EBER status and its association with the overall survival.
Our study carries some limitations. Because of its retrospective nature, some clinical and follow-up data might be missing; however, the characteristics of our hospital's medical records minimize the loss of information. In addition, the prospective studies published to date are scarce because PTLD is an uncommon complication and, thus, our cohort has the best cost-effective design to analyze this disorder. On the other hand, our series had no PTLD cases in heart, lung or bowel transplant recipients and there was only one bone marrow transplant patient and no information could be obtained for these subpopulations.
It is important to consider that pathologists in our country have little access to EBER in situ hybridization (ISH) methods. Although the EBV status in all our samples could be determined by the EBER ISH, this technique—the gold standard for tissue analysis—tends to be replaced by immunohistochemistry (IHC) for EBV proteins (such as LMP1 and EBNA-1/2), which is not the methodology of reference because of the false-negative rate.20
On the other hand, several studies performed gene expression profiling, in which there seems to be a role for cytotoxic immune antiviral signaling in EBV+ vs. EBV- cases, which would explain the modification of the microenvironment in germinal center cells in the presence of EBV+.21,22 These studies detected an increase in the immunotolerance signal (PDL1, IDO1) in EBV+. As we have checkpoint inhibitor drugs, this approach may be useful also in the PTLD. Unfortunately, the increase in graft-versus-host disease with this type of drug makes it necessary to evaluate the safety for this type of patient.20
ConclusionThe PTLD incidence was similar to that of previous series and the EBER did not appear as a relevant factor in our patient survival. The immunological characterization of the tumor microenvironment in the PTLD by complex gene expression profiling and identification of target pathways can be of vital importance to stratify patients adequately and better design therapies in the future.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.