The treatment of elderly multiple myeloma (MM) patients with autologous stem cell transplantation (ASCT) is a controversial procedure. Most clinical trials evaluating the safety and efficacy of ASCT have primarily included patients younger than 65 years.
Design and methodsThis was a retrospective analysis of patients with MM who underwent ASCT between 2008 and 2018. Patients at or over 65 years were compared with patients under 65 years. We analyzed treatment-related mortality (TRM), response rate, progression-free survival (PFS) and overall survival (OS).
ResultsTwo hundred and twenty-one patients were included: 50 patients at or over 65 years, (median age 68 years), including 7 patients over 70 years and 151 patients under 65 years, (median age 57 years). No differences were found in the neutrophil and platelet engraftment, median days of hospitalization and life support requirement during the hospitalization period for the ASCT. No statistically significant differences were found in the incidence of TRM between both groups at 100 days post-transplant (2% vs. 2.9%, p = 0.322). The ASCT improved complete response and stringent complete response rates (44% vs. 37%, p < 0.001). Survival was not modified by age: after a median follow-up of 53 months, the estimated PFS rates at three years were 63% and 60% (p = 0.88) and the OS rates at five years were 75% and 74% (p = 0.72), respectively.
ConclusionsOur data suggest that the ASCT is feasible in selected elderly patients with MM over 65 years of age, achieving response and survival rates similar to those of younger patients.
Multiple myeloma (MM) is a plasma cell neoplasm, which represents 13% of malignant hematological diseases.1 The incidence of MM is related to age. In a multicenter observational study conducted in Latin America, the median age at diagnosis was 61 years, with 50.2% of patients between 60 and 80 years.2
In newly diagnosed multiple myeloma (NDMM), patients younger than 65 years, the standard treatment includes high-dose chemotherapy (HDT) with melphalan (MEL), followed by autologous stem cell transplantation (ASCT).3,4 Several randomized trials demonstrated that this procedure prolonged progression-free survival (PFS), with contradictory results in the overall survival (OS),5,6 having averages of 25–30 months and 50–55 months, respectively. These results were confirmed in a meta-analysis in which the benefit was exclusive to the PFS.7
There is no consensus on the age limit to offer an ASCT, being 65 the age most commonly used.8 In clinical practice, the ASCT is now offered more frequently to fit elderly patients, irrespective of their chronological age. The benefit of the ASCT in elderly patients is controversial.9,10 Aging causes a decrease in organ function and drug metabolism, with an increased risk of toxicity and treatment-related mortality (TRM). Furthermore, there is little evidence on the duration of treatment response and survival in elderly patients. The main reason is the low representation of this group of patients in randomized clinical trials.11 In randomized clinical trials in patients over 65 years of age, which compared the transplantation strategy with conventional treatments, Palumbo et al. reported the superiority of the ASCT,5 while Facon et al. could not demonstrate this benefit.12 Prospective series evaluated the safety and efficacy in elderly patients with encouraging results, using both full and reduced doses of MEL, in terms of PFS or TRM.13 No differences were found in retrospective comparisons of elderly with patients under 65 years.14
The world population is aging, with a progressive increase in the incidence rate of the diagnosis of MM.15 The approval of monoclonal antibodies in patients with NDMM16,17 is an attractive treatment strategy in this group of elderly patients that could compete with the ASCT. The objective of this study was to evaluate the safety and efficacy of the HDT-ASCT in MM patients at or over 65 years and compare these results with patients under 65 years transplanted in the same period in the real-world clinical practice.
Material and methodsPatients and treatmentThis was a retrospective analysis of patients with MM who received HDT-ASCT at Hospital Italiano de Buenos Aires in Argentina. All patients included in this study were NDMM patients. They were referred to the transplant center for an ASCT. Some patients in this study received more than one line of treatment at induction due to refractory disease or suboptimal response. All consecutive patients who received the ASCT from January 2008 to December 2018 were included. Patients with plasma cell leukemia and light chain amyloidosis were excluded. The data were extracted from the electronic medical record and collected on a standardized case report form. Patients undergoing a follow-up at another center were contacted by telephone for follow-up verification. Patients received the induction treatment according to national treatment guidelines at the time of diagnosis.18 The mobilization was performed with the granulocyte colony-stimulating factor (G-CSF) in all patients, with or without prior chemotherapy. The MEL dose was established in the pre-transplant evaluation according to the criteria of the evaluating physician. The standard supportive care was performed and all patients received G-CSF from day 5 after the ASCT until the neutrophil count recovery according to our center guidelines.
DefinitionsPatients were stratified into two groups according to their age at the time of the ASCT: patients at or over 65 years old (Group 1) and patients under 65 years old (Group 2). The International Staging System (ISS) was used for risk stratification.19 Unfavorable cytogenetic risk was defined as the presence of deletion 17p (del17p), deletion 1p, gain 1q or amplification 1q and translocation t (4; 14) or t (14; 16) by fluorescent in situ hybridization (FISH) study, del13q or monosomy 13 in the cytogenetic study or complex karyotype. The standard cytogenetic risk was defined as a normal karyotype or the absence of unfavorable cytogenetic risk. The cytogenetic risk was unknown when the karyotype or FISH data were not available. The HCT-CI (Hematopoietic Cell Transplantation-specific Comorbidity Index) was used for the evaluation of comorbidities20: patients were stratified according to score 0, 1 to 2 or 3 or more. The MEL dose was considered full dose at 200 mg/m2 (MEL200), or reduced dose at 140 mg/m2 (MEL140). The neutrophil engraftment was defined as a neutrophil count over 500/mm3 for 3 consecutive days and the platelet count was defined as platelet count over 20,000/mm3 without transfusion requirement for 7 consecutive days.
Endpoints and statistical analysisThe primary endpoint was TRM and secondary endpoints were response rate, PFS and OS. To evaluate the safety of the ASCT, hospitalization days, days until engraftment of neutrophils and platelets, TRM, 100-day mortality and 1-year post-transplant and life support requirement during hospitalization (orotracheal intubation and mechanical respiratory assistance, hemodialysis requirement and vasopressors) were recorded. To assess the efficacy of the ASCT, the disease status at the time of the ASCT and at day 100 post-ASCT was recorded according to the response criteria of the International Myeloma Working Group (IMWG).21 In patients whose response to treatment was evaluated with the IMWG Minimal Residual Disease (MRD) criteria, the absence of phenotypically aberrant clonal plasma cells was reported as MRD- by bone marrow flow cytometry with a minimum sensitivity of 1 in 105 nucleated cells.21
The Wilcoxon Rank Sum test was used to compare medians between continuous variables and the Chi2 test, to compare categorical variables. The TRM was defined as mortality without relapse or progression after the ASCT, while mortality at day 100 and 1-year post-transplant was defined as death from any cause within 100 days and one year, respectively, after the ASCT. The incidence of the TRM was obtained by the Gray Test. The OS was defined as the time in months from the ASCT to death from any cause or loss to follow-up and the PFS, as the time in months from the ASCT to relapse or disease progression, death from any cause or loss to follow-up. The probability of the OS and PFS were calculated using the Kaplan–Meier method and compared using the log-rank test. The hazard ratio (HR) was calculated according to the multivariate Cox regression model. A univariate analysis of independent predictors associated with the OS and PFS was performed using the Mantel-Cox test (log-rank test) and a multivariate analysis using the Cox proportional hazards model of factors associated with the OS and PFS.
Values of p < 0.05 were considered statistically significant. A confidence interval (CI) was defined at 95%. The data were analyzed using the STATA v13.1 and EZR software. This study was evaluated and approved by the Ethics Committee of the Hospital Italiano de Buenos Aires under Protocol N° 5129.
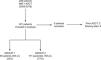
ResultsWe identified a cohort of 226 potential patients diagnosed with MM between January 2008 and December 2018 who had received the HDT-ASCT, of whom 221 patients were included under the selection criteria (Figure 1). The demographic and disease characteristics are summarized in Table 1.
Patients characteristics and treatment.
Abbreviations: ISS: international staging system; PI: proteasome inhibitor; IMiDs: Immunomodulatory Drugs; HCT-CI: Hematopoietic Cell Transplantation-Comorbidity Index.
*For the ISS variable, the percentage per group was considered excluding the missing data, which was 20 (9%): 6 in Group 1 and 14 in Group 2.
The patients were divided into two groups: Group 1 (≥ 65 years, n = 50, median age 68 years, range: 65 - 74) with 7 patients older than 70 years (14%) and Group 2 (< 65 years, n = 171, median age 57 years, range: 32 - 64). Group 1 had more patients with advanced disease (ISS III: 50% vs. 29%, p = 0.01) and a higher frequency of severe comorbidities (HCT-CI ≥ 3: 28% vs. 13%, p = 0.023) (Table 1). The cytogenetic risk was unknown in 70% of patients, with no difference between both groups. The majority of patients (90%) received treatment with a proteasome inhibitor before ASCT. The regimens were VCD (bortezomib, cyclophosphamide and dexamethasone) in 158 patients (72%), VTD (bortezomib, thalidomide and dexamethasone) in 31 patients (14%), VD (bortezomib and dexamethasone) in 7 patients (3%), RVD (lenalidomide, bortezomib and dexamethasone) in 2 patients (1%) and TD (thalidomide and dexamethasone) in 23 patients (10%).
A conditioning regimen with MEL140 was used in 12 patients (24%) in Group 1 and 9 (5%) in Group 2 (p < 0.001) (Table 2). No significant differences were found between the two groups in the median days of hospitalization, days until neutrophil and platelet engraftment and life support requirement during the hospitalization period for the ASCT, except for the hemodialysis requirement (6% vs. 1%, p = 0.03).
Transplant data.
During the follow-up, 64 patients died after the ASCT, 73% due to disease progression: 30% (n = 15) in Group 1 and 29% (n = 49) in Group 2 (p = 0 0.85). Of these, seven patients died during the first 100 days after the ASCT, 2 patients in Group 1 (4%) and 5 in Group 2 (3%) (p = 0.7), all due to complications during treatment. Deaths were secondary to septic shock in three patients, respiratory failure due to pulmonary infections in three patients, and intracranial hemorrhage in one patient with severe thrombocytopenia.
The incidence of 100-day TRM in the Group 1 and Group 2 were 2% (95%CI: 0.2% - 9.3%) and 2.9% (95%CI: 1.1% - 6.3%), respectively (p = 0.32). In Group 1, deaths occurred in patients over 65 and up to 70 years old, with no deaths in patients older than 70 years. No events occurred beyond 100 days. Thirty-one patients (14%) received consolidation after the ASCT with the same induction regimen, 7 patients in group 1 and 24 patients in group 2 (14% vs. 14%, p = 0.81). Eighty-four received maintenance treatment, 23 patients in Group 1 and 61 patients in Group 2 (48% vs. 37%, p = 0.15). The regimens were lenalidomide in 57 patients (69%), bortezomib in 10 patients (12%), lenalidomide and bortezomib in 7 patients (8%), ixazomib in 2 patients (2%) and thalidomide in 8 patients (9%).
Response rates to treatment at transplantation and at day 100 after the ASCT are shown in Figure 2. The overall response rate (ORR: complete response, very good partial response or partial response) were 100% in both groups at day 100 after the ASCT. The ASCT improved the complete response rates (CR/sCR) in both groups, being 44% in Group 1 and 37% in Group 2 (p < 0.001) (Table 3). Among the 82 patients who presented the CR/sCR, the MRD was analyzed in 9 patients, 4 patients having MRD- and 5, MRD+. In the rest (89%), it was not possible to obtain this parameter (because the test was not performed or the adequate sensitivity in its determination was not achieved). There were no differences in both groups (p = 0.74).
In the univariate analysis, the response rate to treatment at transplantation and maintenance therapy presented a significant prognostic value for the PFS and OS, whereas previous treatment with a proteasome inhibitor was only associated with the OS. Age was not significantly associated with the PFS or OS (Table 4). In the multivariate analysis, the response rate to treatment at transplantation and in previous treatment with a proteasome inhibitor were associated with a better OS, while age at transplant had no impact. For the PFS, only the response rate to treatment at transplantation showed statistical significance. Furthermore, maintenance therapy was independently associated with a better PFS and OS (Table 5).
Univariate analysis of prognostic factors (log rank).
Abbreviations: ISS: international staging system; ASCT: autologous stem cell transplantation; PI: proteasome inhibitor; HCT-CI: Hematopoietic Cell Transplantation-Comorbidity Index.
Multivariate analysis of prognostic factors (proportional hazards model).
Abbreviations: CR: complete responde; sCR stringent CR; VGPR: very good partial response; PR: partial response; SD: stable disease; ASCT: Autologous Stem Cell Transplant; PI: proteasome inhibitor.
After a median follow-up of 53 months (interquartile range (IQR) 25 - 80), data on disease status and survival were collected in April 2022. The median PFS was 48 months (95%CI: 40 - 63) and median OS was 140 months (95%IC: 107 - not reached). No statistically significant differences were found in the median follow-up between the two groups (48.5 months, IQR 26 - 78 vs. 54 months, IQR 24 - 82; p = 0.59). Elderly patients (Group 1) had a median PFS of 48 months (95%CI: 32 - 58), compared to the median of 53 months (95%CI: 39 - 66) for younger patients (Group 2), with a 3-year PFS of 63% (95%CI: 47 - 75) and 60% (95%CI: 53 - 68) [HR 1.03, 95%CI 0.67 - 1.59, p = 0.88], respectively. The median OS was 107 months in Group 1 (95%CI: 74 - not reached) and 140 months in Group 2 (95%CI: 106 - not reached), while the 5-year OS in Group 1 was 75% (95%CI: 58 - 86) and 74% (95%CI: 66 - 80) in Group 2 [HR 1.11, 95%CI 0.62 - 1.98, p = 0.72] (Figure 3).
Patients who received maintenance had better survival rates in both groups. In patients who received maintenance, the median PFS in Group 1 was not reached, similar to Group 2 (95%CI: 46 - not reached), whereas for those who did not receive maintenance, the median PFS was 39 months (95%CI: 23 - 53) vs. 40 months (95%CI: 19 - 49), respectively [HR 0.35, 95%CI 0.22 - 0.56, p < 0.001]. The median OS for patients who received maintenance was not reached in Group 1 and Group 2 (95%CI: 84 - not reached). In contrast, for patients who did not receive maintenance, the median OS in Group 1 was 107 months (95%CI: 70 - not reached) vs. 140 months (95%CI: 83 - not reached) in Group 2 [HR 0.32, 95%CI 0.15 - 0.69, p = 0.003].
DiscussionThis retrospective study evaluated the safety and efficacy of the ASCT in patients with MM. Compared with patients younger than 65 years, similar rates of the TRM, PFS and OS were found. These data are encouraging because the prognosis of patients with MM has improved, both in young and elderly patients.22
The HDT-ASCT treatment has improved both the response and survival of MM patients, including the use of novel agents.23 Although in recent years there have been a greater number of patients undergoing the ASCT with a better survival, particularly in elderly patients,11,24 the percentage of patients who access the ASCT worldwide based on real-life data is less than expected.25 In a retrospective multicenter study of Latin America, 32.7% of patients with MM underwent ASCT, however the percentages varied between countries from 69% in Argentina to 3% in Chile.2
Elderly patients are more likely to have treatment-related toxicity.26 Our analysis found no significant difference between the two groups in transplant-related safety. Although we have not performed a specific toxicity analysis in both groups, proper selection of older patients is effective in reducing toxicity without compromising treatment outcomes. There were also no statistically significant differences in the TRM at 100 days, with acceptable results in both groups. Age alone is not the only parameter to decide if the patient is a candidate for the ASCT. The elderly comprise a heterogeneous population with different degrees of vulnerability. An evaluation should be performed to estimate their aptitude for transplantation,27 which includes a measurement of frailty and comorbidities.28
The Comprehensive Geriatric Evaluation (CGA) is used to assess the functional and global health status of elderly patients, allowing the selection of therapy more appropriately.29 In MM patients, frailty can be assessed using the IMWG-frailty Score,28 Revised Myeloma Comorbidity Index (R-MCI)30 and Mayo Frailty Index,31 while the most appropriate scores for the ASCT are the R-MCI and HCT-CI.32 In our experience, the HCT-CI was used systematically in the pre-transplant evaluation, only evaluating frailty with a CGA in 10 of 50 patients (20%). Patients with frailty probably had complications during the induction treatment that made it impossible for them to access, or be candidates for, the ASCT. These tools should be incorporated into clinical practice for the assessment of frailty and comorbidities. Since 2018, we have performed a CGA in all patients older than 65 years prior to chemotherapy treatment, helping us make decisions regarding the ASCT. Although there may be a selection bias in our group of patients, admission to the Transplant Unit is offered to all fit patients who have their comorbidities compensated. All patients up to 75 years of age are evaluated to determine if they are eligible for the ASCT. Additionally, some patients are referred only for the procedure. The evaluation of comorbidities was used to define the MEL doses, adjusting mainly in those patients with serum creatinine over 2 mg/dl or clearance under 30 ml/min. In this study, the decision on whether the patient was suitable for the ASCT was based on the treating physician and standard operating procedure manuals. An ongoing debate is whether less fit patients could be included for the ASCT with adjusted doses of MEL.
Most of the evidence for the ASCT in elderly patients were from retrospective studies.9,11,33 A balance should be found in the conditioning dose to maintain a deep response without increased toxicity or TRM. Garderet et al., in a prospective study, found no difference between MEL200 and MEL140, with a better PFS rate for patients who received full doses.27 In our cohort, the 100-day TRM did not show differences in both groups (p = 0.322), in which 60% of the patients had comorbidities. The rates are similar to those in other studies.14,34,35
Although the ASCT is controversial in the elderly in terms of efficacy, in a prospective study by GIMEMA, the ORR was 94% (with 75% ≥ VGPR), with a median PFS of 48 months.36 Palumbo et al. showed CR rates of 38% in patients aged 65 to 75 years after induction with bortezomib plus ASCT.37 In our analysis, only a quarter of the elderly patients received MEL140, with 79% with, or more than, the VGPR and 44% CR/sCR. These results are similar to other studies, in the ORR, VGPR, or over, and PFS rates.34 The PFS and OS did not show differences according to the age of the patients at the time of transplantation in our analysis. Elderly patients obtained the same survival benefit as the young patients and we found that the PFS and OS were not significantly different. The maintenance therapy was shown to improve the PFS and OS,38 which is also observed in our results, but it should be noted that, in Argentina, the national recommendation guidelines have incorporated maintenance since 2015. Although we have incorporated the MRD assessment since 2016, low rates of MRD- were obtained in our results. It is an objective to improve, since it was only evaluated in 10% of the patients that could have been studied.
In this non-standardized retrospective analysis of a highly selected group of elderly patients, several characteristics were comparable between the groups. The median follow-up and baseline characteristics were comparable in both groups, except that the elderly had more advanced stages and a higher frequency of severe comorbidities. The cytogenetic risk could not be assessed in all patients. In the period in which the study was conducted, together with the difficulties in accessing FISH studies in Latin America, only a minority of patients had data available on the cytogenetic risk and most of these studies were conducted without the sorting of plasma cells. Therefore, these data may present a bias in the prognosis of patients. Despite the retrospective design of this study, the majority of patients received treatment with novel drugs and almost all patients received a proteasome inhibitor in their induction.
The approval of monoclonal antibodies in patients with NDMM is an attractive treatment strategy in this group of elderly patients that could compete with the ASCT. Access to these drugs and their cost/effectiveness should be a topic of discussion, mainly due to their unequal access among countries and health coverage systems, so it is essential to have real-world evidence comparing both strategies. The ASCT may be a cost-effective strategy for elderly fit patients in middle- and low-income countries, where access to monoclonal antibodies or innovative treatments are non-existent or more restrictive. Herein, we described real-world evidence on the ASCT in elderly patients.
ConclusionsThe improvement in treatment and supportive therapies, together with the adequate selection of patients beyond chronological age, have allowed encouraging results of the HDT-ASCT in elderly patients, comparable to those observed in young patients, with similar rates of safety and efficacy. Chronological age alone is not an exclusion criteria for transplantation and it should be evaluated together with comorbidities and the physical condition of the patients. These parameters are measured objectively, primarily to detect frailty and identify patients who may benefit from the ASCT.
The results presented show real-world data on the HDT-ASCT in elderly patients, with the objective of achieving better responses to treatment and survival. Although the results obtained in this study confirm the results of the larger series, we emphasize that it is a study carried out in a Latin American country, where access to treatment and transplantation are sometimes a challenge. We conclude, based on the reported data, that the HDT-ASCT is a safe and effective procedure in fit patients, regardless of the age presented at the time of the ASCT, achieving results and benefits in the elderly population similar to those in young patients. Future prospective randomized trials with elderly patients are required to determine the real impact of this treatment, compared to the results presented with the new treatment regimens.