Consensus of the Brazilian association of hematology, hemotherapy and cellular therapy on patient blood management
More infoHemostasis plays a critical role in surgical procedures and is essential for a successful outcome. Advances in hemostatic agents offer new approaches to controlling bleeding thereby making surgeries safer. The appropriate choice of these agents is crucial. Volume replacement, another integral part of Patient Blood Management (PBM), maintains adequate tissue perfusion, preventing cellular damage. Individualization in fluid administration is vital with the choice between crystalloids and colloids depending on each case. Colloids, unlike crystalloids, increase oncotic pressure, contributing to fluid retention in the intravascular space. Understanding these aspects is essential to ensure safe and effective surgery, minimizing complications related to blood loss and maintaining the patient's hemodynamic status.
Hemostasis undoubtedly plays a critical and fundamental role in all surgical procedures. Its effective management begins with the pre-operative identification and management of risk factors for increased bleeding, although this article will focus on the intraoperative period, through the application of solid surgical techniques and adequate anesthetic support, establishing the essential pillars for a successful surgical outcome.1 Currently, bleeding control can benefit from innovative solutions, which, together with the skills of the surgical team, contribute to reducing or controlling blood loss.2
The continued advancement of biotechnology has resulted in the development of topical hemostatic agents, offering surgeons a broader arsenal of options to address the challenge of hemostasis. These agents range from absorbable hemostatic solutions, such as gelatins, microfibrillar collagen and regenerated oxidized cellulose, to biologically active options, such as thrombin, biological glues and other combinations of substances.3-8
These advances not only enrich the surgeon's options, but also open new horizons in the treatment of difficult-to-control bleeding. By combining surgical expertise with these innovative solutions, it is possible to achieve more effective management of hemostasis, reducing the risks associated with excessive blood loss and ultimately providing patients with more effective and safer surgery.
Hemostatic agents: exploring options for controlling surgical bleedingThe effectiveness of hemostasis is a critical factor in surgical procedures and continuous advances in medicine have provided a diverse range of hemostatic agents to meet the needs of surgeons.5-7
In the surgical context, a variety of hemostatic agents is available for intraoperative use, each with distinct mechanisms and applications. These agents can be classified as active or passive depending on their action in promoting hemostasis.9-13
Active agentsActive hemostatic agents play a direct role in promoting blood clotting in particular products that contain thrombin, a key enzyme in the clotting process. These products accelerate the conversion of fibrinogen to fibrin promoting the rapid formation of a stable clot. Topical thrombin is frequently used in vascular and liver surgeries due to its effectiveness in local hemostasis.
Passive agentsPassive hemostatic agents, on the other hand, work by creating a mechanical matrix or barrier that aids hemostasis; examples are gels, sponges and absorbable dressings. These materials promote platelet aggregation, providing physical support for clot formation and in so doing accelerate the healing process. They are especially useful in surgical procedures where occlusion of larger areas is necessary, such as liver resection.
Hemostatic agents can also be classified according to three main categories: absorbable, biological and synthetic.
Absorbable agents- 1.
Regenerated oxidized cellulose (Surgicel Original®, Surgicel Nu-Knit®, Surgicel Fibrillar®, Interceed®, Gelitacel®): These products, derived from cellulose, are effective in promoting hemostasis. They are applied directly to the site of the hemorrhage and act by absorbing the blood, forming a gel that helps with clotting. Their gradual absorption by the body facilitates healing.
- 2.
Gelatins (Surgifoam®, Gelfoam®, Gelfilm®, Gelita-spon®, Geli putty®, FloSeal®): Gelatin is a porous material that also promotes hemostasis by absorbing blood. Many of them can be used as vehicles for other hemostatic agents or drugs. FloSeal, for example, is a combination of gelatin and thrombin matrix.
- 3.
Microfibrillar collagen (Instat®, Helitene®, Helistat®, Avitene®, Avitene flour®, Avitene Ultrafoam®, EndoAvitene®, Avitene Ultrawrap®): Microfibrillar collagen promotes hemostasis through its ability to attract platelets and stimulate clotting. These products are known for their biocompatibility and gradual dissolution in the body, facilitating healing.
- 1.
Fibrin sealants (Evicel®, Tisseal®, Crosseal®, Quixil®): When mixed and applied to the site of bleeding, they form a web-like matrix that promotes clotting. They are particularly effective in vascular surgeries and in places where suturing is not appropriate.
- 2.
Topical thrombin (Evithrom®, Recothrom®, Thrombin-JMI®): Topical thrombin is an enzyme that converts fibrinogen into fibrin, promoting clot formation. It can be used alone or in combination with other fibrin sealants.
- 1.
Glutaraldehyde and bovine albumin adhesive (BioGlue®): BioGlue is a synthetic surgical adhesive that adheres to tissues and blood vessels, effectively sealing them. It is often used in cardiac procedures.
- 2.
Cyanoacrylate adhesives (Dermabond®, Omnex®): Cyanoacrylate adhesives are applied directly to the skin and are mainly used to close skin incisions, avoiding the need for sutures in some situations.
- 3.
Polyethylene glycol (CoSeal®): Polyethylene glycol is a synthetic sealant used in heart and vascular surgeries. It helps prevent leaks at anastomoses and sutures.
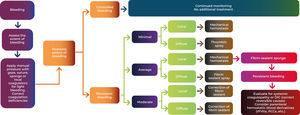
The ideal agent is characterized by its ease of application, proven efficacy, versatility to be used in different surgical scenarios, absence of allergic reactions, complete absorption capacity and affordable cost. The choice of the appropriate hemostatic agent depends on the type of surgery, the type and amount of bleeding, the site of the hemorrhage, and the surgeon's preferences. The large number of available agents can create difficulties for doctors to make up their minds. The figure below as published by Shander et al. presents a proposal for a treatment algorithm, in order to facilitate the surgeon's choice (Figure 1). h Knowledge and proper use of these agents are essential to ensure safe and successful surgery, thereby minimizing the risk of complications resulting from hemorrhage.6,14
Treatment algorithm for topical hemostasis. DIC: Disseminated Intravascular Coagulation; rFVIIa: recombinant factor VII; PCC: Prothrombin complex concentrate. Adapted from Krajewski et al. (2015)15.
- •
Exploration of the crucial role of intravenous fluid therapy in maintaining hemodynamic status during surgery.
Volume replacement is the cornerstone in the treatment of critically ill patients, especially hypovolemic patients. It is known that adequate blood volume is important in optimizing oxygen supply, which, in turn, has an impact on reducing hospitalization, improving perfusion variables and mortality (Table 1).
Volume replacement goals.
| Optimize oxygen delivery |
|---|
| Interrupt tissue hypoperfusion evolution |
| Reduce the organ failure incidence |
| Reduce morbidity and mortality |
| Reduce hospital stay |
Adapted from Samudrala S. (2008)2.
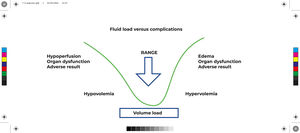
The ideal blood volume curve is represented by a V-shaped curve, where euvolemia is represented by the lowest point of the curve (Figure 2). Both hypovolemia and hypervolemia are associated with negative clinical outcomes. Hypovolemia is associated with metabolic acidosis, renal dysfunction, hypoperfusion among others; hypervolemia, in turn, is associated with damage to the glycocalyx, excess extravascular pulmonary water, interstitial edema and also dilutional coagulopathy.16g
Fluid load versus complications. Adapted from Doherty & Buggy (2012)17.
The most pertinent questions that should be asked before initiating volume replacement in patients are the following four.
During surgery, for example, the body requires a constant amount of oxygen and nutrients to maintain proper cellular function. Intravenous fluid therapy plays a vital role in ensuring that blood flow is maintained at levels sufficient to sustain tissue perfusion. Through adequate fluid administration, it is possible to prevent hypoperfusion that could lead to cellular damage and organ dysfunction.
Individualization and constant monitoringEach patient is unique and fluid needs vary. Therefore, fluid administration must be individualized based on the patient's condition, type of surgery, and estimated fluid loss. Furthermore, continuous monitoring of hemodynamic parameters, such as blood pressure, heart rate and diuresis, is essential to adjust fluid administration appropriately throughout the surgical procedure.
- •
Discussion of tissue perfusion and its dependence on adequate volume replacement
Tissue perfusion is a vital aspect of the health of the body's tissues and organs. During surgery, fluid losses can be significant, whether due to controlled hemorrhages or evaporation and insensible loss. The administration of intravenous fluids aims to replace these losses and maintain circulating volume, ensuring that blood continues to be efficiently delivered to tissues and organs. To achieve adequate volume replacement, the anesthetist must consider several factors, including the type of surgery, the patient's health status, and estimated fluid loss.
Administration of isotonic intravenous solutions, such as lactated Ringer's or saline, is common to effectively restore blood volume. However, individualization is essential, as fluid needs can vary considerably. The adequacy of volume replacement must be closely monitored. Hemodynamic parameters, such as blood pressure, heart rate and diuresis, should be regularly assessed during surgery. This allows for precise adjustments in fluid administration as needed to maintain optimal tissue perfusion.
On the other hand, the development of hypervolemia generally occurs due to excessive volume administration. In many cases, intraoperative fluid is administered to treat hemodynamic instability due to vasodilation, surgical bleeding, myocardial dysfunction or vascular permeability, which often results in postoperative fluid overload.
Although preoperative fasting overnight for approximately ten hours does not significantly reduce intravascular volume, the fasting period is limited to avoid preoperative dehydration. Patients are encouraged to consume clear oral liquids up to two hours before surgery.
Oliguria (urine output <0.5 mL/Kg per hour) is a commonly used indicator of hypovolemia. However, oliguria alone is not a good indicator for fluid administration in patients undergoing anesthesia and surgery. For example, inhalational anesthetics as well as surgical stress can reduce urinary output in patients who are truly euvolemic; this can lead to fluid overload when liquid is administered to treat oliguria.17
Volume replacement: crystalloids vs. colloids- •
Differences between crystalloids and colloids
It is known that the ideal fluid does not exist yet; all fluids, to a greater or lesser extent, can interfere with blood clotting. Excessive fluids, of any nature, can induce dilutional coagulopathy.
In the medical practice, the choice between crystalloid and colloid solutions for fluid replacement is a crucial decision, with significant implications for maintaining homeostasis and treating patients. Both types of solutions have their place and usefulness, but their characteristics and mechanisms of action differ substantially.18-20
Crystalloid solutions - Crystalloids are solutions of electrolytes and sterile water that can be isotonic, hypotonic or hypertonic with respect to plasma. Balanced electrolyte solutions (also called buffered crystalloid solutions) that have an electrolyte composition similar to plasma with the addition of a buffer (e.g., lactate) are most commonly used. Examples include lactated Ringer's solution or Plasmalyte®.
Typically, an electrolyte-balanced crystalloid solution is selected for routine perioperative fluid administration to maintain intraoperative normovolemia. During major surgical procedures approximately 3 mL/Kg/hour is administered to replace sensible and insensible losses and sustain the basal metabolic rate. Additionally, fluid boluses (typically 250 mL) are administered in volume-responsive patients to optimize intravascular volume and replace lost blood with crystalloids on a volume basis of 1.5:1.0. Solutions containing dextrose are avoided due to the supposed adverse effects of hyperglycemia.
Administration of a large volume of normal 0.9 % saline solution is avoided as it has been associated with hyperchloremic acidosis. The risk of other adverse outcomes, particularly acute kidney injury, has been associated with saline solution in several observational and randomized studies.15 However, results are not consistent in patients who do not receive large volumes of saline solution 21 or who are not seriously ill.22
Colloid solutions - Colloids are derived from human plasma (e.g., human albumin, fresh frozen plasma [FFP]) or semisynthetic preparations (e.g., hydroxyethyl starch [HES], gelatins). Colloids can be dissolved in isotonic saline or in a solution with a balanced electrolyte concentration similar to plasma.
Some clinicians prefer to use colloids in selected patients or situations in an attempt to expand microvascular volume with minimal capillary leak in fluid-responsive patients, thereby minimizing the total amount of fluid administered and the formation of edema. For example, during blood loss, colloids may be administered on a 1:1 vol basis. Administration of 20 % albumin results in an expansion of plasma volume that lasts into the postoperative period.
However, evidence that colloidal solutions are superior to electrolytically balanced crystalloid solutions is scarce.23 In general, it is recommended to minimize the use of colloids, as they do not provide significant hemodynamic benefits compared to crystalloids. When a colloid is selected to expand microvascular volume, albumin is used instead of hydroxyethyl starches.
Albumin - Human serum albumin is available in 5 and 25 percent solutions. In some parts of the world, human serum albumin is available in 4 and 20 percent solutions. Five percent human albumin has a volume effect (i.e., the percentage of administered fluid that remains intravascular) of 70 %, whereas 25 % albumin solution is isosmotic with plasma. In Brazil, the human albumin available in the National Health Service is 20 %.24 Human albumin is pasteurized and does not transmit any known infectious disease.
Hydroxyethyl starches (HES) - HES solutions are synthetic colloids, identified by three numbers corresponding to their concentration, molecular weight, and molar substitution (i.e., the average number of hydroxyethyl groups per unit of glucose). Due to concerns regarding renal toxicity and effects on hemostasis, the administration of HES solutions is restricted in Europe and North America.
A 2018 systematic review of critically ill patients with medical and surgical diagnoses noted a higher incidence of renal replacement therapy in those who received HES solutions compared to those who received crystalloids.23 However, the data is not consistent. A 2022 meta-analysis of randomized trials noted that intraoperative intravascular volume replacement with HES during open abdominal surgery was not associated with an increased risk of acute kidney injury 30 days after surgery compared with the use of crystalloid solutions. 25
Because HES products impair platelet reactivity and decrease circulating plasma concentrations of coagulation factor VIII and von Willebrand factor, administration may result in weakened clot formation and increased transfusions of blood products, including FFP, cryoprecipitate, and platelets compared to other fluid options. A 2018 systematic review of randomized trials of critically ill patients noted a higher incidence of transfusion in those who received HES solutions compared to those who received crystalloids.23
It is known that the differences between crystalloids and colloids go beyond their molecular characteristics; although colloids exert greater colloid-osmotic force due to their greater oncotic power and the ability to attract water intravascularly, this benefit was not translated into clinical relevance when compared with crystalloids. 26–28 Despite of improving blood volume with smaller volumes, colloid solutions were more associated with different adverse effects; pruritus, anaphylactic and anaphylactoid reactions have been reported, especially with first-generation colloids. However, even later generation colloids have been associated with renal dysfunction and altered hemostasis in critically ill patients. 29 Below are Table 2 shows the main changes in the colloid during coagulation.
Specific anticoagulant effect of colloids.
Adapted from Kozek-Langenecker (2015)29.
Gelatins - Gelatins are not used in the United States because of their short duration of action (2–3 h) and because of rapid excretion in the urine, possible clotting effects and a relatively high incidence of anaphylaxis. Gelatin is used in some countries, such as Brazil, because it is cheap and has a volume effect of 70–80 %.
The composition of the fluid can also interfere with coagulation in critically ill patients. It is well known that vigorous volume replacement with 0.9 % saline solutions is more associated with hyperchloremic metabolic acidosis due to its low pH and high chlorine content. Acidosis, in turn, is known to make clotting difficult as it worsens the enzymatic reactions necessary to maintain homeostasis and hemostasis.
It is still important to remember that balanced crystalloid solutions, although they have a pH closer to the physiological pH, do not contain the calcium ion. This ion, which is the IV cofactor of coagulation, needs to be replaced in critical situations involving significant bleeding or in patients who are being transfused, as the citrate in blood bags reduces serum calcium levels.
As previously stated, the ideal amount of fluid is important not only for coagulation, but also for the maintenance of organic functions in general. Excess fluids, of any nature, can worsen the coagulopathy due to the dilution of clotting factors, in addition to protease dysfunction. 30,31
Finally, we know that patients go through different resuscitation phases throughout their hospitalization. In general, acute patients (for example: polytrauma or dehydrated patients and those with intra-abdominal conditions) require more volume at the time of their arrival, when volume replacement is essential to change their clinical outcomes, and less volume throughout their hospitalization. After restoring the homeostasis of organic systems or microcirculatory variables, the fluids become part of the context of a maintenance and rebalancing phase. Excess fluids reported in these phases are associated with harmful effects such as those described above.
Choosing a fluid management strategyThe intraoperative fluid management strategy and selection of non-invasive or invasive monitoring are based on expected blood loss and the likelihood of non-bleeding fluid changes (e.g., from open body cavities and wounds) during the planned surgical procedure. Other factors that influence these decisions include patient comorbidities (e.g., anemia, congestive heart failure [CHF], chronic obstructive pulmonary disease [COPD]) and planned postoperative disposition (e.g., home, ward, intensive care unit).
Minimally/moderately invasive surgeryFor most relatively brief, minimally or moderately invasive surgeries with planned early postoperative ambulation, 1–2 liters of a balanced electrolyte solution is administered in procedures that will not cause significant fluid changes or blood loss. This volume of fluid is administered during surgery over a period of 30 min to two hours. This empirical but limited administration of fluids for less invasive outpatient surgery addresses mild dehydration caused by preoperative fasting and is associated with less risk of postoperative nausea and vomiting or pain compared with controls receiving minimal fluids. In patients with a history of CHF or COPD, the liquid must be administered with caution.
Major invasive surgeryRestrictive strategy (zero balance)32-34 - For major invasive surgery with expected blood loss <500 mL, a restrictive zero-balance approach is employed that minimizes fluid administration, especially if no invasive monitoring of hemodynamic parameters is planned. With this approach, only the fluid lost during surgery is replaced using the following strategies:
- •
During the intraoperative period, patients receive a balanced electrolyte crystalloid solution administered at a rate of approximately 3 mL/Kg/hour to replace sensitive and insensitive losses and sustain metabolic rates.
- •
In case of blood loss, additional fluid may be given. Studies suggest that the ideal crystalloid-to-blood volume ratio is approximately 1.5:1.0, and that the ideal colloid-to-blood ratio is 1:1.
- •
Crystalloid “preloading” prior to neuraxial block or induction of general anesthesia is avoided.
- •
Replacement of non-anatomical “third space” losses is avoided, as evidence suggests that this practice does not bring benefits and can cause morbidity.
- •
Avoid extremely deep anesthesia as this may result in hypotension with treatment using unnecessary additional fluid.
- •
Administration of a total volume of balanced electrolyte solutions that modestly exceeds zero fluid balance is appropriate in patients with evidence of hypovolemia.
For major invasive surgeries with anticipated significant blood losses (e.g., >500 mL) and/or fluid changes, it is recommended to employ a goal-directed approach to fluid administration using one or more invasive hemodynamic parameters to achieve the pre-specified goal. For example, intra-arterial waveform tracing may be used for automated measurements of pulse pressure variations (PPV) or stroke volume variation, or visually estimated or manually calculated PPV or systolic pressure variations, in order to determine responses to fluid bolus (typically 250 mL increments).
Practical recommendations for hemostasis and volume replacementIntraoperative hemostasis:
- 1.
Minimizing Blood Loss: Start by taking a proactive approach to minimizing blood loss. This includes careful surgical techniques, use of precise electrocautery and proper blood vessel control.
- 2.
Continuous Monitoring: Maintain constant monitoring of bleeding during surgery. This can be achieved through visual assessment (using a bleeding scale such as the Validated Intraoperative Bleeding [VIBe] Scale7,8) and the use of devices such as a blood scale.
- 3.
Hemostatic agents: Be familiar with available hemostatic agents and choose them wisely. Keep in mind that not all situations require the same agent. Topical agents, such as hemostatic sponges or fibrin sealants, may be particularly useful in surgeries with the potential for bleeding.
Intraoperative volumetric replacement:
- 1.
Prior assessment: Perform a thorough patient assessment prior to surgery to determine volume status and predict estimated fluid losses. This helps customize your fluid replacement strategy.
- 2.
Individualization: There is no one-size-fits-all approach to volumetric replacement. Consider age, comorbidities, type of surgery, and estimated losses when customizing your fluid replacement plan.
- 3.
Crystalloids vs. colloids: Understand the differences between crystalloids and colloids. Crystalloids are often used as the first choice due to their availability and low cost, but colloids may be necessary in specific situations to maintain colloid osmotic pressure.
- 4.
Hemodynamic monitoring: Use hemodynamic monitoring, such as invasive blood pressure, to assess the patient's response to volumetric replacement and make adjustments as needed.
- 5.
Fluid Limitation: Avoid excessive fluid administration, which can lead to volume overload. Use the Goal-Directed Therapy approach to set specific hemodynamic goals and optimize fluid replacement according to those goals.
Hemostasis and intraoperative volume replacement play a central role in the concept of Patient Blood Management (PBM), which aims to optimize patient care and reduce the need for blood transfusions. By effectively controlling hemostasis during surgery and applying adequate intraoperative fluid therapy, healthcare professionals can minimize the risk of excessive blood loss, transfusions and associated complications. This not only improves patient safety but also results in saving medical resources.
Intraoperative Phase – Intraoperative hemostasis and volume replacement