Follicular lymphoma (FL) is one of the most common types of Non-Hodgkin Lymphoma (NHL) which originates from B-cells.1 Generally it has an indolent clinical course, although histological transformation to aggressive lymphoma may occur in 25-35 % of cases, and therafter the prognosis has been reported to be poor. Transformation to histiocytic sarcoma may also be seen but very rarely.1
Langerhans Cell Histiocytosis (LCH) is a rare neoplasm of the clonal neoplastic proliferation of Langerhans cells with unknown etiology. It has a clinical course ranging from indolent isolated lesions to life-threatening systemic disease. LCH may very occasionally be associated with malignant neoplasms, especially lymphomas.2 The most commonly seen associated lymphoma is classical Hodgkin Lymphoma (HL) whereas the association of other NHL subtypes has been reported in extremely few cases.3 The impact of this association on survival and biological behaviour of the disease is still unclear due to its rarity, and therefore investigation in this field has not been sufficient.4
To date, the co-existence in the same lymph node of LCH with FL has been reported in one case and with mantle cell lymphoma in another case.1,5 However, there is no case in which LCH and FL have been determined in the same parotid gland (PG). The case is here reported of a patient with concomitant FL grade II and LCH which developed in the same PG.
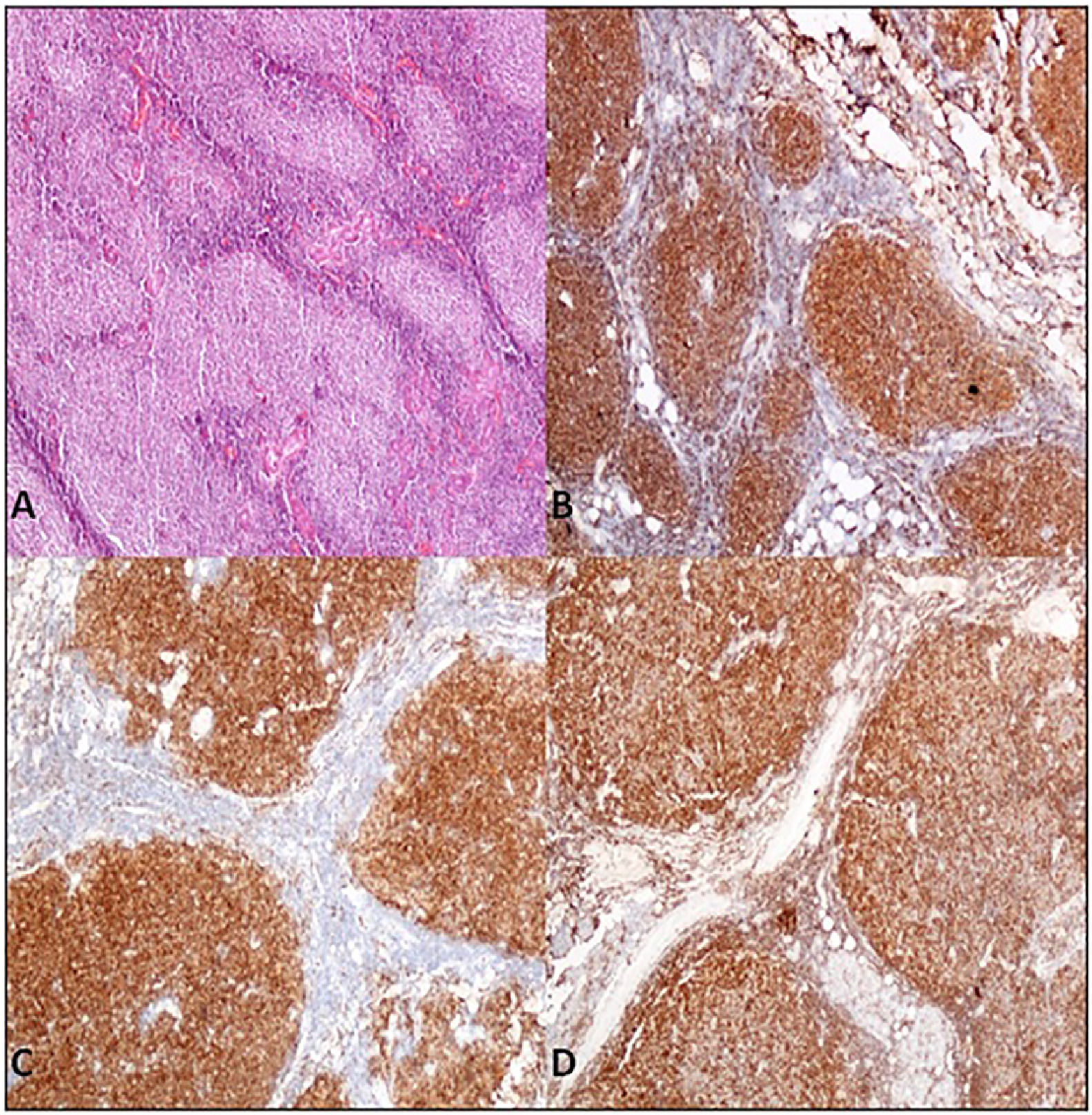
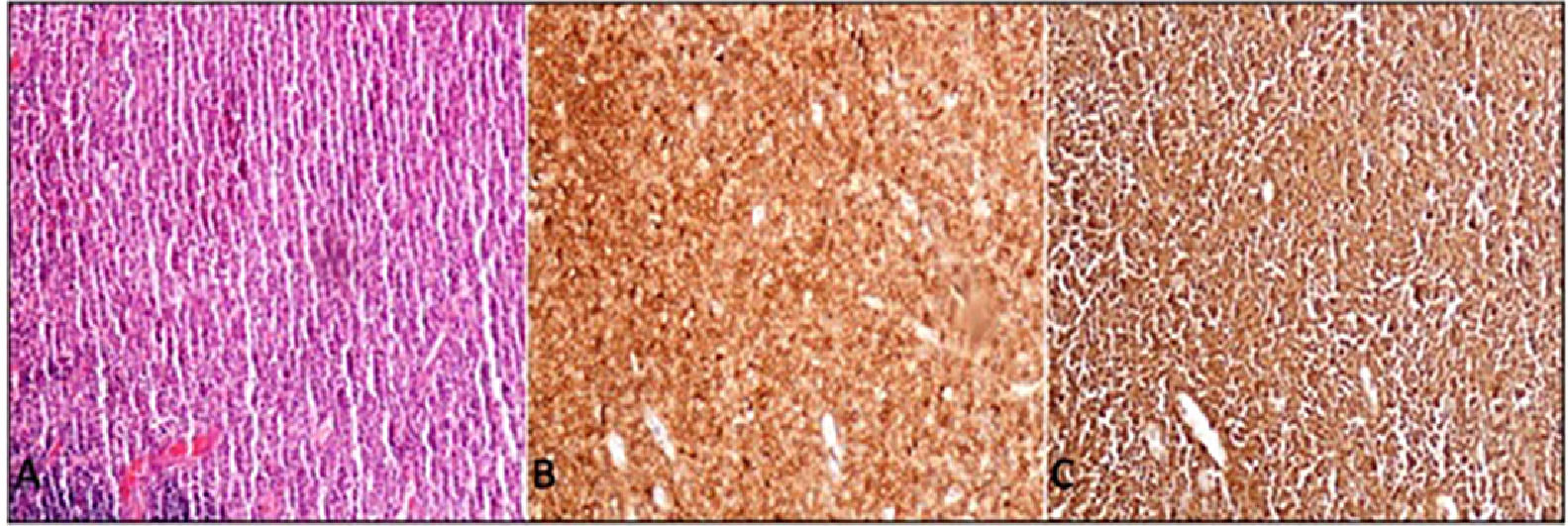
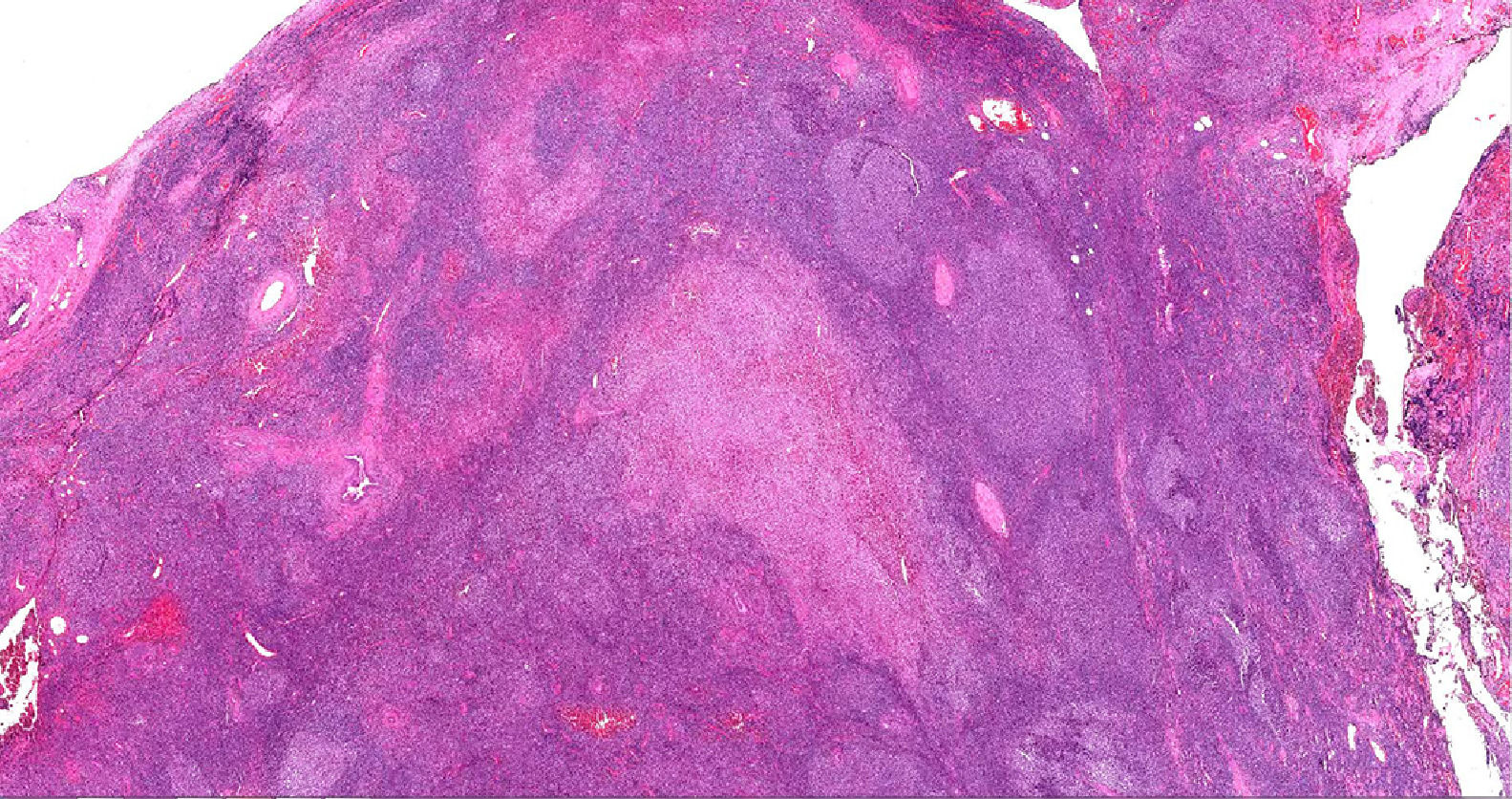
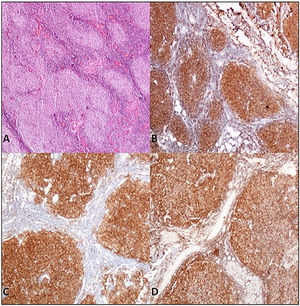
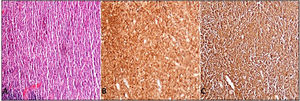
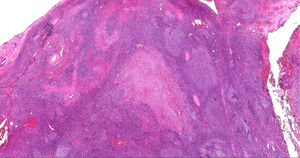
Case presentationA 62-year-old male patient presented at our centre with complaints of swelling on the right PG. Past history revealed that this swelling in that region had been ongoing for the past 4 years. There were no accompanying B symptoms. Complete blood count, erythrocyte sedimentation rate (12 mm/h) and lactate dehydrogenase level (194 u/L) were all found to be normal. Computed tomography (CT) of the neck, thorax and abdomen revealed no organomegaly or lymphadenopathy. Cranial magnetic resonance imaging was normal. There was no disease involvement in the aspiration and biopsy of bone marrow. An excisional biopsy was performed from the right PG. The morphology of the specimen showed complete architectural effacement by a nodular lymphoid proliferation of small lymphocytes with elongated and cleaved nuclei (Figure 1A) in addition to a well defined nodule 4 mm in diameter. Regions with acidophilic cytoplasm, pale clefted nuclei, inconspicous nucleoli, and large atypical cells showed easily detectable nuclear bodies (Figure 2A). The tumor cells in the regions with follicular structure showed positivity for CD20 (Figure 1B), BCL2 (Figure 1C), and CD10 (Figure 1D), and negativity for cyclin D1 and SOX11. Follicular pattern was demonstrated with the follicular dendritic cell marker CD23. It was ultimately evaluated as follicular lymphoma. According to the WHO 2017 classification, it was graded by counting the absolute number of centroblast in 10 neoplastic follicules, expressed per high- power microscopic field. Eight centroblasts were counted in 10 HPF areas, which is consistent with a grade 1-2 follicular lymphoma. The tumor cells in the regions without follicular structure showed positivity for CD1a (Figure 2B), and S-100 (Figure 2C), and negativity for CD163, confirming LCH. Both FL and LCH within the same tissue was shown on Figure 3 (H&E, x40). Total bone scintigraphy revealed non-specific lesions demonstrating no involvement of the diseases. According to all these results, the patient was diagnosed as concurrent grade 2 FL and LCH originating from the right-side PG. As there was no indication of treatment for both diseases, follow-up of the patient was started without any medication.
A. Parotis gland involved by follicular lymphoma composed of homogeneus nodules (H&E,X40) B. Tumor cells of follicular lymphoma showed positivity for immunohistochemistry of CD20 (CD20/peroxidase stain,X40) C. Bcl-2 (Bcl-2/peroxidase stain,X40) D. CD10 (CD10/peroxidase stain,X40).
LCH is defined as a clonal proliferation of Langerhans cells (LCs) which express CD1a, Langerin (CD207) and S100 protein. Birbeck granules can be demonstrated on ultrastructural examination.2 Although there have been many advances in molecular biology and genetics, the etiology of LCH is still unclear. Some investigators have suggested that LCH is a myeloid neoplasia with inflammatory properties. Genetic mutations and chromosomal abnormalities have recently been reported to play an important role in the development of this disease.6
Histiocytic disorders are heterogeneous diseases with a heterogenous clinical course. It is sometimes difficult to classify the subgroups, and there is ongoing debate whether LCH originates from a different clone or is a reactive and/or therapy related disease.7 Prognosis is generally good in cases with localized LCH, although the association with other malignancies confers a poorer outcome. The optimal therapy is still not clear.7 It has been previously reported that LCH can develop before, after, or synchronous with other tumor types, especially malignant lymphomas.2 The association with other malignancies especially lymphoproliferative disorders has been rarely reported whereas the association with HL is well known. In a study of 91 cases of LCH, an association with malignant lymphoma was determined in 39 cases.8 Another study showed 40 cases with association of LCH and HL.3 In contrast, Pina-Oviedo et al. reported 7 cases of LCH associated with lymphomas including 5 classic HL, 1 MCL and one angioimmunoblastic T-cell lymphoma, and this was the first report of LCH associated with MCL.6
An association or co-existence of FL and LCH is very rare. In 2018, a case of FL and HCL development in the same lymph node was reported by Shimono J. et al.1 Both tumor cells showed positivity of BCL6, IgH clonality and some identical amino acid sequences in direct sequence analysis of DNA. That case is important in demonstrating both association and developmental origin of those two distinct entities. In the current case, FL and LCH development were detected in the same PG. The tumor cells in the regions with follicular structure showed immunohistochemical staining consistent with the diagnosis of FL grade 2 whereas the tumor cells in the regions without follicular structure were positive for CD1a, and S-100, and negative for CD163 confirming Langerhans cell histiocytosis. Since we could not perform clonality in our case, as a limitation of the study, we cannot make a suggestion on this issue. However, in a study supporting these association, West et al. demonstrated a clonal relationship between FL and Langerhans cell neoplasm using genetic and hybridization studies.9
In conclusion, LCH is a rare neoplasm of the clonal neoplastic proliferation of Langerhans cells. Although rare, LCH can be associated with various malignant neoplasm. To the best of our knowledge, this is the first case in which FL and LCH developed concurrently in the same PG. This finding could be an important basis for future research on the development and relationships of this group of neoplasms.
Compliance with ethical standardsAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.