An intracellular parasite of mononuclear phagocytes, mainly distributed in the bone marrow and the spleen, causes visceral leishmaniasis. Complete blood count (CBC) reveals the poorly understood pathogenesis of anemia, leukopenia and thrombocytopenia. Our study aimed to compare the CBC with bone marrow cytomorphological features and their association with clinical outcomes to clarify this relevant issue.
MethodsThe CBC and bone marrow of 118 patients were described by two hematologists and compared to check their association with each other and mortality.
ResultsPeripheral cytopenias were common findings, particularly anemia, as seen in almost all patients. No relationship was found between values of hemoglobin, neutrophils and platelet count with fatal outcomes. The bone marrow was normocellular in 61.9% of the cases. Dysplasia figures were frequent and 49.1% of the samples had dysgranulopoiesis. Additionally, erythroid hyperplasia was found in 72% of the patients with severe anemia. Patients with reduced bone marrow cellularity, erythroid hypercellularity and dyserythropoiesis seem to have a riskier disease.
ConclusionThe study results suggest that the bone marrow of patients with visceral leishmaniasis manifests a reactional pattern to the inflammatory event, thereby modulating cytokines and other colony growth factors. This compensatory response may be dysplastic and ineffective and generate peripheral cytopenias of varying intensity. Further studies are needed to clarify the signaling pathways involved, which may be used as therapeutic tools in the future.
Visceral leishmaniasis (VL) or kala-azar is caused by the intracellular Trypanosomatidae protozoa Leishmania infantum and L. donovani. Both are transmitted by the bite of several sandfly species and infect macrophages and monocytes, principally in the spleen, liver, bone marrow, lymph nodes and other organs. L. donovani is endemic throughout Northeast India, Bangladesh and Nepal, where it used to reach the highest worldwide incidence, but it has also been responsible for devastating epidemics in East Africa, especially in Ethiopia, Sudan and South Sudan. L. infantum has a broader distribution, but a lower incidence, ranging from China to the Middle East, the Mediterranean Basin and Latin America, with the highest incidence in Brazil.1 Another striking difference is that of the affected species; the VL, caused by L. donovani, is found among humans, while the L. infantum infects a broader range of mammals, from humans to domestic and sylvatic canids, rodents and lagomorphs.2
The disease starts with a high fever, anemia and wasting, with hepatosplenomegaly developing quickly. Loss of appetite, dyspnea, generalized edema, jaundice and diarrhea are other important signs and symptoms that usually indicate a poorer prognosis. The disease can sometimes run for months, but some patients develop bacterial infections and hemorrhagic phenomena that quickly lead to death.3 Both parasites cause important opportunistic infections in patients with acquired immunodeficiency syndrome and other immunosuppressive conditions in endemic areas.4
The diagnosis is based on the identification of the parasite and its products or through non-specific tests. Parasites are identified by the bone marrow, spleen, or lymph node aspiration, followed by direct observation and culture and detection of Leishmania molecules by polymerase chain reaction, or other techniques.5 Antibody immunoassays are very sensitive, but not as specific as parasitological or molecular diagnosis, and are valuable in resource-deprived endemic areas.6
Several observations suggest that most clinical and laboratory findings are the result of systemic inflammation, in which proinflammatory cytokines play important roles.7 Interleukin 6 (IL-6) increases the risk of bleeding by stimulating tissue factor expression,8 thereby initiating the coagulation cascade, while another relevant role is to trigger acute phase response (APR). The APR alters the hepatic synthesis of many proteins, such as in the decreasing of albumin synthesis and the increasing of that of hepcidin.9 The complete blood count (CBC) in VL typically shows the so-called pancytopenia, with red blood cell, hemoglobin, leukocyte, neutrophil and platelet reduction. These changes may not be due to the direct action on bone marrow hematopoiesis, but to systemic inflammation, such as iron deprivation, disseminated intravascular coagulation, or hypersplenism.
Our study aimed to compare the CBC with bone marrow cytomorphological features and their association with clinical outcomes to clarify this relevant issue.
MethodsStudy populationThis study included patients admitted at the Instituto de Doenças Tropicais Natan Portella in Teresina, Brazil, which is a reference center for disease treatment. The study population consisted of patients of both sexes and all ages, symptomatic, untreated and with no VL history, who were submitted to bone marrow puncture to search for Leishmania. Patients who met the inclusion criteria were enrolled in the study on a non-probabilistic and consecutive basis.
Patients with either serological or parasitological laboratory confirmation of VL were included. Only 118 of the 141 patients initially enrolled in the database were included in the present study. Individuals whose slides could not be analyzed due to staining wear were excluded, as well as those who received a substitutive diagnosis of another disease with similar clinical manifestations. This study did not consider the diagnoses by the PCR or immunochromatographic test because they had not been authorized for VL diagnosis at that time.
ProceduresAll patients underwent anamnesis and physical examination by physicians involved in the research within the first 48 hours of admission, regardless of previous disease confirmation. The blood count was requested upon admission and bone marrow aspirate samples to complement the laboratory investigation were provided after the blood count results within the first 48 hours. Blood samples were collected by peripheral vein puncture in sterile and dry bottles, or bottles containing anticoagulants (ethylenediaminetetraacetic acid and 3.2% sodium citrate). Screening for the human immunodeficiency virus (HIV) was requested after pre-test guidance and obtaining verbal consent, following the proposed guidelines by the Ministry of Health.10
The indirect immunofluorescence reaction for Leishmania was performed with the indirect immunofluorescent test (IFI Human Leishmaniasis Bio-Manguinhos, Rio de Janeiro, Brazil). The parasitological evidence of VL was based on the visualization of amastigote forms on a bone marrow smear or promastigote form detection by cultured bone marrow or blood. The slides were immediately sent to the laboratory after each patient bone marrow aspiration, where they were fixed and stained using the May-Grunwald-Giemsa or panoptic staining technique. Two independent hematologists (MAFC and AMRDP) evaluated the bone marrow aspirate. All slides were initially observed at lower power (10× objective) to visualize the presence of particles, trails and cells in the medullary smear. Subsequently, the most representative part of the slide, with adequate staining, was selected for observation at higher magnification (40× and 100× objective) to guarantee a quality morphological assessment.11
The bone marrow biopsy histology was impossible because the method is not routinely performed at the hospital, although it is the most recommended technique for confirming cellularity, instead of aspirates and smear, and the study was retrospective in design.12 Thus, the observation of precursors and other typical bone marrow cells in adequate amounts for the age was considered normal cellularity. Additionally, descriptive analysis was performed based on the presence or absence of morphological alterations in each series. The presence of atypia was considered when 10% or more of the lineage was in question. The number of megakaryocytes was evaluated at a lower power (10× objective), being considered as normal (1 cell per 1 – 3 fields), increased (> 2 per field), or decreased (1 in every 5 – 10 fields).13
Data analysisData tabulation and statistical analysis were performed using Epi Info® Version 7.1.4 (Division of Integrated Surveillance Systems and Services, National Center for Public Health Informatics and Centers for Diseases Control and Prevention) and Stata R version 15.1, College Station, TX, USA. Quantitative data were presented as absolute values, proportions, means and confidence intervals.
The odds ratio (OR) was used to calculate the relationship between variables. The chi-square test or Fisher's exact test was used to analyze the differences between dichotomous variables when data were sparse. The Student's t-test was used to compare differences between means of continuous variables of independent samples with normal distribution. The One-way analysis of variance was used to test for differences in the means of normally distributed dependent variables versus categorical independent variables with two or more categories.
Ethical considerationsThe study was conducted following the fundamental precepts of the National Health Council-CNS Resolution 196/96, which deals with the Guidelines and Norms for Research Involving Human Beings, which was still in effect at the time of the data collection from the reference study for this research.
All patients included in the study or their guardians signed an informed consent form. This study made no changes in therapeutic recommendations, causing no interference with medical decisions, because all stages of the study were observational. Authorization was obtained for the use of the stored biological material and database consultation generated by the original study, considering the term of responsibility for the information, which was assumed as a commitment by the researcher. The original work on which this research was based was submitted to the Research Ethics Committee of the Federal University of Piauí and approved on December 14, 2005 under number 0116/05.
ResultsStudy populationThis study analyzed bone marrow aspirates and blood counts from 118 hospitalized patients, including 71 (60.2%) males and 47 (39.8%) females. The mean age was 14 years (range: 3 months to 86 years). The presence of comorbidities was observed in 63 (53.4%) patients. Bacterial infections were the most common complication in 16.9% of the cases. Bleeding alone was present in 13.5% of the individuals and is also found in association with combined infection. The HIV co-infection was observed in 5 (4.2%) patients and 8 (6.8%) patients died in the hospital.
The blood cell countAnemia was found in 97.5% of the patients. The mean hemoglobin was 7.4 g/dL (range: 2.4 – 12.6 g/dL). Severe anemia, characterized by hemoglobin levels of <7.0 g/dL, was observed in 36.4% of the patients. Leukopenia, with a blood count of <4.0 × 109/L was seen in 80 (67.8%) participants. The mean leukocyte count was 3.2 × 109/L (range: 0.54 × 109/L to 12.0 × 109/L). Thrombocytopenia was seen in 73.7% of the participants, and 27 (22.9%) of all the individuals evaluated in the study evolved with counts of <50 × 109/L. The mean platelet count was 109 × 109/L (range: 14 × 109/L to 290 × 109/L). No relationship was found between the hemoglobin, severe neutropenia and platelet count with progression to a fatal outcome (data not shown, p > 0.05).
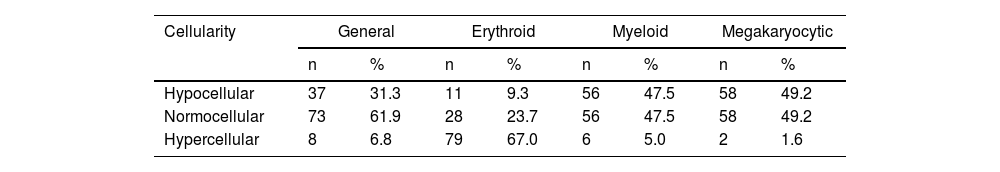
Bone marrowThe bone marrow was normocellular in 61.9% of the patients, reduced cellularity was observed in 31.4% and 8 had bone marrow with increased cellularity. Regarding each cell lineage, most patients had erythroid hypercellularity (67.0%). However, this pattern was not detected in the myeloid and megakaryocytic sectors, in which nearly half of the patients had these hypocellular lineages (Table 1).
Distribution of bone marrow cellularity according to the cell lineage of patients with visceral leishmaniasis, Piauí, December 2006 to August 2007 (n = 118).
| Cellularity | General | Erythroid | Myeloid | Megakaryocytic | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Hypocellular | 37 | 31.3 | 11 | 9.3 | 56 | 47.5 | 58 | 49.2 |
| Normocellular | 73 | 61.9 | 28 | 23.7 | 56 | 47.5 | 58 | 49.2 |
| Hypercellular | 8 | 6.8 | 79 | 67.0 | 6 | 5.0 | 2 | 1.6 |
Source: Prepared by the authors from study data.
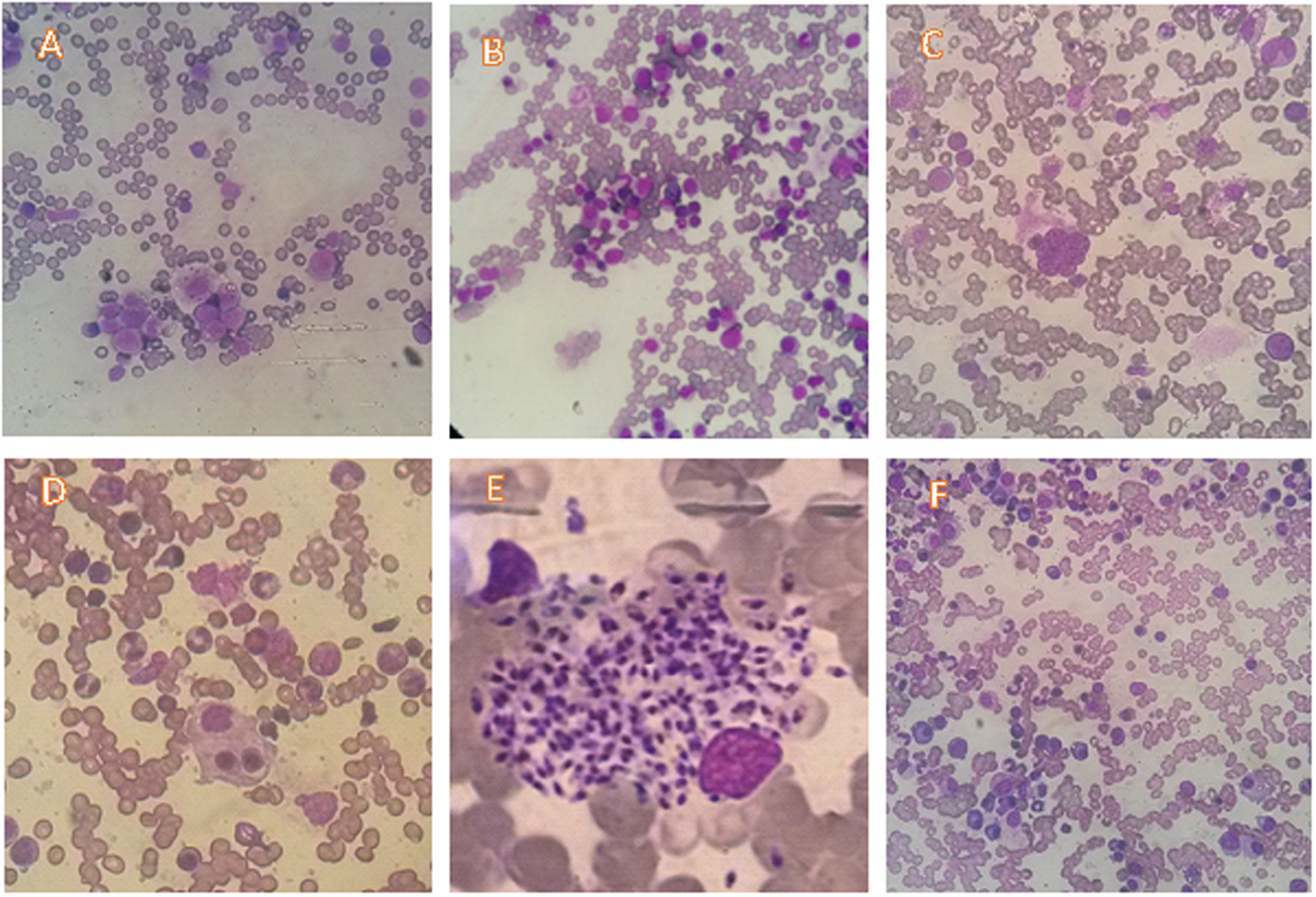
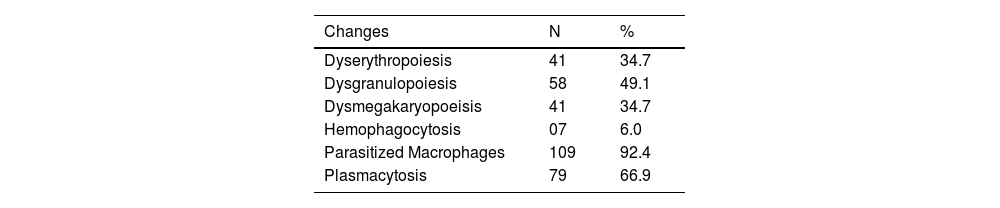
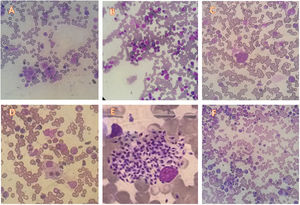
The most prevalently found changes were plasmacytosis and multilineage dysplasia (Table 2). The presence of mild degrees of hemodilution was verified in 22 patients, but that did not compromise the slide evaluation as a whole. Figure 1 shows examples of the most typical aspects of the bone marrow of the participants.
Cytomorphological changes of bone marrow in patients with visceral leishmaniasis, Piauí, December 2006 to August 2007 (n = 118).
| Changes | N | % |
|---|---|---|
| Dyserythropoiesis | 41 | 34.7 |
| Dysgranulopoiesis | 58 | 49.1 |
| Dysmegakaryopoeisis | 41 | 34.7 |
| Hemophagocytosis | 07 | 6.0 |
| Parasitized Macrophages | 109 | 92.4 |
| Plasmacytosis | 79 | 66.9 |
Source: Prepared by the authors from study data.
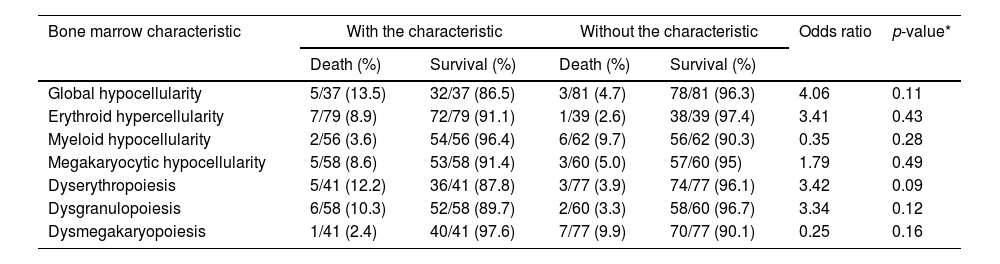
The small sample size limited statistical power, while increasing the probability of a type II error. Thus, patients with reduced bone marrow cellularity were more likely to die, (OR = 4.0) although the p-value was greater than the stipulated value limit of 0.05. The same was repeated with erythroid hyperplasia (OR = 3.4), but with a much higher p-value. Myeloid hypoplasia may have a protective effect, but megakaryocytic hypoplasia was not related to death. Conversely, erythroid and myeloid dysplasia indicated the risk of death more consistently with an OR of >3 and a p-value closer to the conventional limit (Table 3).
Bone marrow characteristics of patients with visceral leishmaniasis according to clinical outcomes (survival or death), Piauí, December 2006 to August 2007 (n = 118).
Source: Prepared by the authors from study data. *Fisher's exact test.
Erythroid hyperplasia in the bone marrow was observed in 72.1% of the patients who developed severe anemia in this sample. Individuals who had hemoglobin levels of >7.0 g/dL at admission had this characteristic with a frequency of 64.0%. However, this finding was not associated with an increased risk of mortality. No visible association was found between myeloid cellularity and neutropenia. The mean counts were higher in patients who demonstrated megakaryocytic hypercellularity among patients with thrombocytopenia. However, no statistically significant association was found between bone marrow findings and platelet counts (data not shown).
DiscussionThis study mainly aimed to correlate the blood cell count findings with those of the bone marrow. Almost all participants had some degree of anemia. Two-thirds had leukopenia and one-quarter had marked thrombocytopenia. In general, the bone marrow revealed normocellularity. Meanwhile, most presented erythroid hypercellularity according to the cell lineage. Almost half of the patients had myeloid and megakaryocytic hypocellularity. No blood count was associated with mortality in the sample, but patients with reduced cellularity in the bone marrow had a riskier disease, as were those with erythroid hypercellularity, dyserythropoiesis and dysgranulopoiesis. Interestingly, patients with myeloid hypoplasia and dysmegakaryopoiesis had a lower risk of death, but this did not present statistical significance.
This study of patients admitted to a tropical disease referral hospital is representative of the population with VL caused by L. infantum in Brazil. It affected more males than females and more children than adults. The proportion of participants with HIV was lower than that expected for Brazil and the mortality was comparable to the national average.
Blood cells were in the same pattern routinely seen in patients with anemia, leukopenia and thrombocytopenia.2,14,15 Almost 100% had anemia, and a significant proportion had severe anemia, with hemoglobin of <7.0 g/dL, indicative of red blood cell transfusion. Kager and Rees carefully studied the nature of VL anemia in 47 patients in Kenya16 and described anemia as microcytic and hypochromic, with low iron concentration, attributed to the concept of anemia with chronic inflammation. Today, VL anemia is indeed mediated by the hepatic hormone hepcidin, which acts by trapping iron in splenic and hepatic macrophages and duodenal enterocytes, through the enzyme ferroportin inactivation.17 Hepcidin is an acute phase protein secreted after the stimulus conferred, mainly by IL-6, which explains the inexorable improvement after treating this infectious and inflammatory condition. Hence, iron supplementation is not necessary for disease treatment, despite the slow recovery from anemia after treatment.18
This study revealed no clear association between leukopenia and thrombocytopenia with VL anemia, despite being frequent. The degree of the contribution of hypersplenism to VL is unclear since other phenomena participate in the processes, although its role in neutropenia and thrombocytopenia is recognized.19
The granulocyte colony-stimulating factor (G-CSF), which exerts its effects through the G-CSF receptor, is the main physiological granulopoiesis regulator. Neutrophils maintain G-CSF receptors at high levels on the surface of immature cells and the CXC chemokine receptor 4 (CXCR4) is also expressed at low levels on the cell surface of mature neutrophils. The major ligand for the CXCR4 is stromal-derived factor 1 (SDF-1) and the CXCR4-SDF-1 signaling axis retains neutrophils within the marrow environment.20 Animal model studies revealed that the CXCR4 deletion in murine myeloid cells results in an increased neutrophil release which reinforces the role of the CXCR4-SDF-1 interaction in regulating neutrophil exit from the bone marrow.20
Systemic inflammation is associated with increased neutrophils and multiple mediator counts. However, acute neutrophil mobilization from the bone marrow may require the coordinated actions of cytokines and chemokines.20 Other authors revealed that patients with active VL have a higher proportion of circulating CD4 T-cells expressing the CXCR4 than healthy controls.21 Thus, further studies are needed to explain neutropenia and decreased cellularity of myeloid lineage in VL. We hypothesize that alterations in the expression of G-CSF receptors and transcription factors associated with the CXCR4-SDF-1 axis overexpression could explain this finding.
Previous animal model studies suggest that thrombocytopenia involves coordinated alterations in the megakaryocyte demarcation membrane system, interferences in signaling pathways of thrombopoietin possibly mediated for inflammatory cells and increased levels of splenic clearance due to platelet membrane changes.22
Several bone marrow morphology changes were observed in this sample, but none are exclusive to the disease or have not already been described in previous studies, including dyserythropoiesis, dysgranulopoiesis and dysmegakaryopoeisis.23,24,25 Bone marrow biopsy evaluation of patients with VL indicates erythroid hyperplasia as a very common finding and is accompanied by frequent abnormal erythroblasts. Animal model studies suggest that anemia in VL is associated with increased serum erythropoietin levels, the erythropoiesis inhibition caused by the apoptosis induction of immature cells mediated by the expression of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and the erythroblast differentiation blockage due to the decreased erythroid differentiation gene expression.26 The presence of plasmacytosis in two-thirds of the patients is noteworthy, which might be due to the polyclonal hypergammaglobulinemia of VL that seems dependent on the B cell differentiation and antibody production.27
Severe anemia, neutropenia and thrombocytopenia were revealed as risk factors for death in a meta-analysis, as well as several other clinical and laboratory elements.28 However, the present study did not identify any association between peripheral blood counts and death, probably due to the limited statistical power, with only eight deaths. Additionally, the analysis of the association between myelodysplasias and death suggested substantial effect sizes, despite the lack of statistical association.
The study had three main limitations, including the small sample size, the subjectivity of interpreting bone marrow smear slides and the variation in cell concentration due to the bone marrow aspiration process, which is difficult to standardize and may have exaggerated the type II error.
ConclusionIn conclusion, the study results suggest that the bone marrow of a patient with VL manifests a reactional pattern to the inflammatory event, thereby receiving modulation of cytokines and other colony growth factors. This compensatory response may be dysplastic and ineffective and may generate peripheral cytopenias of varying intensity. Further studies are needed to clarify the involved signaling pathways that may be used as therapeutic tools in the future.
FundingsThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.