Culturing bone marrow mesenchymal stem cells (BM-MSCs) is a key point in different fields of research, including tissue engineering and regenerative medicine and studies of the bone marrow microenvironment. However, isolating and expanding murine BM-MSCs in vitro has challenged researchers due to the low purity and yield of obtained cells. In this study, we aimed to evaluate five different protocols to culture murine BM-MSCs in vitro.
MethodsAll protocols were based on the adhesion capacity of BM-MSCs to the tissue culture plastic surface and varied in the types of plate, culture media, serum, additional supplementation and initial cell density. Flow cytometry analysis was used to investigate lineage purity after expansion.
ResultsThe expression of CD45 and CD11b was detected in the cultures generated according to all protocols, indicating low purity with the presence of hematopoietic cells and macrophages. The cellular growth rate and morphology varied between the cultures performed according to each protocol. Cells cultured according to protocol 5 (8 × 107cells/plate, Roswell Park Memorial Institute (RPMI) culture medium during first passage and then Iscove's Modified Delbecco's Medium (IMDM) culture medium, both supplemented with 9% fetal bovine serum, 9% horse serum, 12µM L-glutamine) presented the best performance, with a satisfactory growth rate and spindle-shape morphology.
ConclusionOur results point out that the purity and satisfactory growth rate of murine BM-MSC cultures are not easily achieved and additional approaches must be tested for a proper cell expansion.
Hematopoietic stem cells (HSCs) are responsible for continually repopulating the blood system throughout the lifetime of an individual due to their capacity of self-renewal and of differentiating into mature blood cells.1–3 The equilibrium between the quiescence and expansion of HSCs is maintained by the bone marrow niche, which is composed of heterogeneous cell populations.2 Mesenchymal stem cells (MSCs) are major components of this microenvironment and are responsible for the release of cytokines, mediators and growth factors.3
The MSCs are multipotent cells existing in different tissues and, as stem cells, they have the potential of self-renewal and of giving rise to different cell types, such as adipocytes, chondrocytes, osteoblasts and neurons.4,5 Therefore, the MSCs represent a central source for cell therapy in regenerative medicine and tissue engineering, with diverse therapeutic applications, such as the treatment of degenerative diseases, immune-based disorders, graft-versus-host disease and infertility.6
In cancer, it has been well accepted that cells from the local microenvironment interact with tumor cells and participate in different steps of carcinogenesis.7 This interplay between normal and neoplastic cells has also been reported to contribute to the pathophysiology of hematological malignancies, such as myelodysplastic syndromes, acute myeloid leukemia and myeloproliferative neoplasms-like disease,2,8 being a potential therapeutic target.9 Studies using murine models indicate that those malignancies present microenvironment disruption.10 However, the role of the niche in these diseases remains unclear. Moreover, knowledge regarding the role of the niche in normal conditions remains scarce.8,11
In this scenario, culturing murine MSCs from bone marrow (BM) has emerged as a model for in vitro studies of MSC functions. Human MSCs are identified according to specific surface markers, adhesion capacity and ability to differentiate in vitro.4,12 However, unlike human bone marrow mesenchymal stem cells (BM-MSCs), culturing murine BM-MSCs has been challenging due to the low yield of isolated cells and presence of hematopoietic cells.5,13 Different approaches have been tested in the attempt to achieve an efficient method to harvest and obtain a pure culture of BM-MSCs.14 However, there are few studies comparing the efficiency of these protocols.5,14,15 Therefore, the purpose of the present study was to evaluate five different protocols of culturing murine BM-MSCs. The protocols were based on preferential adhesion of BM-MSCs to tissue plastic, in the attempt to avoid intense manipulation or frequent changes in conditions that could alter the biological properties of these cells. The cell morphology, growth rate and purity were examined during expansion.
Materials and methodsAnimal modelMale animals of the C57BL/6 mouse strain (The Jackson Laboratory) were chosen in consideration of their availability and robustness.16 In addition, the C57BL/6 mouse strain is usually chosen for the development of animal models for hematological diseases.17 Animals were bred and maintained at the University of Campinas (UNICAMP) and housed four per cage. Briefly, environmental conditions were a temperature-controlled condition (21°C ± 2°C), humidity (55 ± 5%) and a 12h:12h light-dark circadian cycle, with access to food and water ad libitum. All the experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and the study was approved by the Institutional Animal Experimentation Ethics Committee (CEUA 4399-1/UNICAMP).
Isolation and culture of murine BM-MSCsMice were euthanized by cervical dislocation, cleaned with 70% ethanol and placed in a 100-mm cell culture dish. Bone marrow cells were harvested from the two femurs of each animal and plated according to each protocol (Table 1). Culture media was supplemented with 100 U/mL penicillin and 100 µg/mL streptomycin (Sigma-Aldrich). Information regarding the type of plate, culture media, serum (fetal bovine serum (FBS) or horse serum (HS)) and additional supplementation for each culture is detailed in Table 1.
Details of the five protocols used to culture murine BM-MSCs.
DMEM: Dulbecco's Modified Eagle Medium; RPMI-1640: Roswell Park Memorial Institute; IMDM: Iscove's modified Dulbecco's Medium; FBS: Fetal Bovine Serum; HS: horse serum.
For protocols 1 and 2, experiments were performed with two serum concentrations (10% or 20%).
Protocol 1: After dissection of the 6-week-old mice, the femurs were macerated with 5mL of phosphate buffered saline (PBS) and the cells were isolated using a strainer. All the cells obtained from each mouse were re-suspended in the proper medium and plated in a 25cm2 flask. Half of the medium was replaced every 7 days.
Protocols 2,3,4 and 5 used the flushing method to harvest the bone marrow cells. Briefly, the flushing method consists in cutting the ends of the bone and removing the perivascular cells from the bone marrow using a syringe and a needle containing culture media in the bone cavity.5
Protocol 2: After the bone marrow flushing, the cells of 6- to 8-week-old mice were cultured in a flask at a concentration of 1 × 107cells/25cm2. Half of the medium was replaced every 7 days until the cultures reached approximately 80% of cell confluency.
Protocol 3: This protocol was similar to protocol 2, except for the initial cell density (2 × 327107cells/25cm2) in 20% HS.
Protocol 4: This protocol was based on the procedures described by Meirelles et al.18 with minor modifications. Briefly, bone marrow cells of a 16-week-old mouse were cultured at a concentration of 1.75 × 107cells/well in a 6-well plate. The medium was completely changed after 72h and then frequently changed, based on visual indications of consumption, until the cultures reached approximately 80% confluency.
Protocol 5: The cells were cultured based on Peister et al.15 with minor modifications. Briefly, the murine bones were inserted into adapted centrifuge tubes and centrifuged for 1 minute at 400g to collect the bone marrow. After centrifugation, residual cells were collected according to the flushing method. All the cells from each mouse were suspended (RPMI-1640, supplemented with 9% FBS, 9% HS and 12µM L-glutamine) and plated in a 175cm2 flask. Non-adherent cells were removed after 24 hours and the medium was completely replaced every 4 days. After 4 weeks, the cells were plated in a new 175 cm2 flask (passage 1). After passage 2, 50 cells/cm2 were expanded in the IMDM medium (supplemented with 9% FBS, 9% HS and 12µM L-glutamine) (Table 1). The medium was replaced every 3 to 4 days until the new expansion. The culture was terminated on the 6th passage.
Morphologic analysis and immunophenotypingThe cell growth and morphology were observed weekly under a light microscopy. The cell purity was evaluated by flow cytometry at the end of the cultures using the following conjugated antibodies: APC-conjugated anti-mouse CD31 (Biolegend), CD44 (Biolegend); PE-conjugated anti-mouse Ter119 (BD Biosciences) and CD11b (BD Biosciences); PerCP-conjugated anti-mouse CD45 (Biolegend) and PECy7-conjugated anti-mouse Sca-1 (e-Biosciences).
ResultsThe initial characteristics of the cultured cells were similar among all tested protocols. The first week was characterized by the presence of small adherent and non-adherent cells. Non-adherent cells remained circular, whereas adherent cells started to spread and become slightly elongated. The cellular growth rate, morphology and phenotype differed between the five tested protocols after the first media change.
Cells cultured according to protocol 1 with 20% FBS were more elongated and presented longer protrusions compared with the cells cultured with 10% FBS (Figure 1A). Both cultures (10% and 20% FBS) were maintained for 7 weeks after the selection of adherent cells, without reaching the needed confluency to be sub-cultured and the purity could not be analyzed by flow cytometry due to the insufficient number of cells. The variation in the serum concentration induced no changes in the cellular growth rate (Table 2).
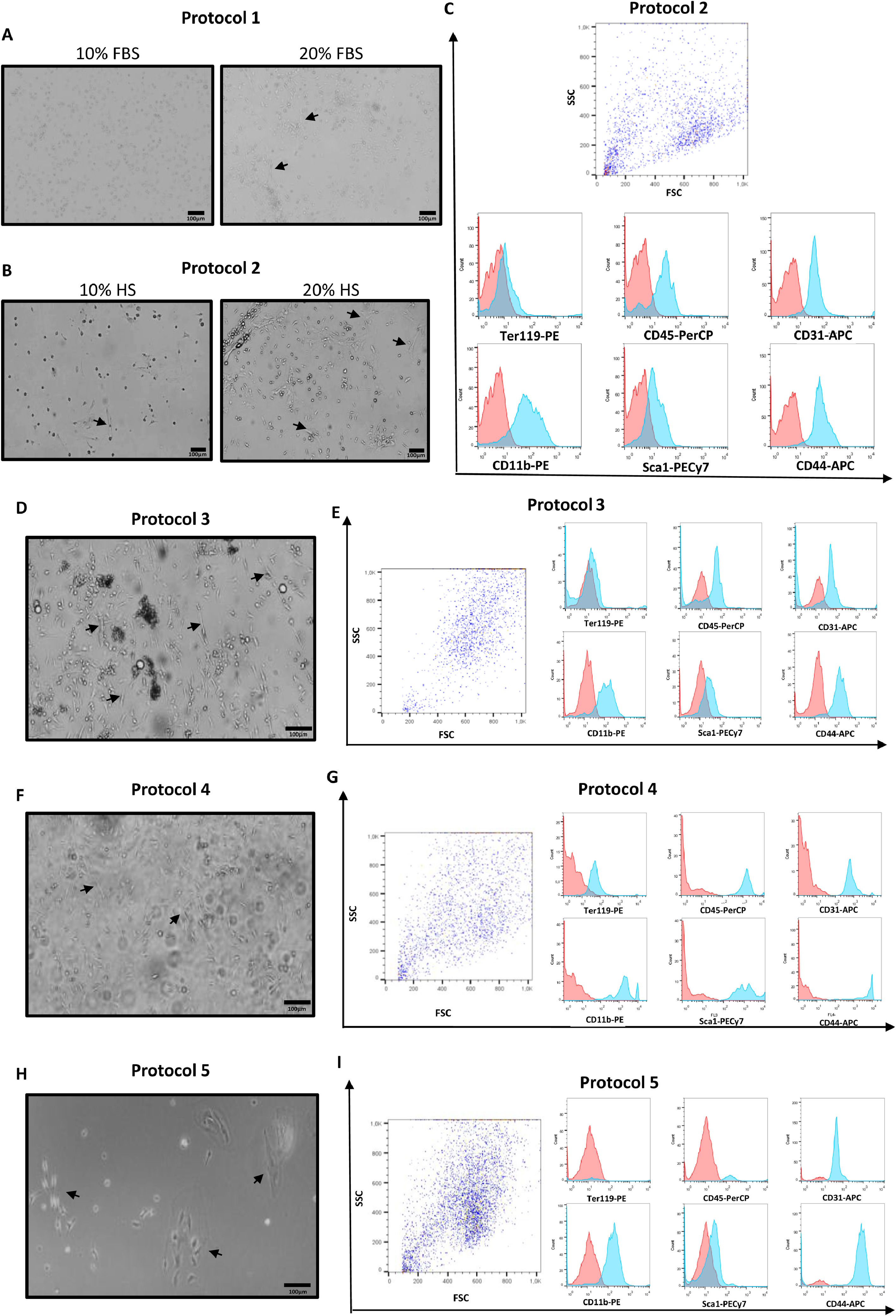
Morphology and purity analysis of murine BM-MSCs cultured according to different protocols. (A) Cells cultured according to protocol 1 after 21 days of plating. Cells cultured with 10% FBS were rounder and smaller, compared with cells cultured with 20% FBS, which were more elongated, arranged in groups and presented more protrusions (arrows). These cells did not reach the necessary confluency to be analyzed by flow cytometry. (B) Cells cultured according to protocol 2 after 10 days of culture with 10% HS or 20% HS supplementation. Observe that the cultures containing 20% HS presented more elongated cells with a fibroblastic-like cell shape (arrows) and higher confluency, whereas the 10% HS condition generated fewer cells, of which most were round and dark indicating unviability (arrows). (C) The panel of cell surface markers consisted of antibodies anti-Ter119, anti-CD45, anti-CD31, anti-CD11b, anti-Sca1 and anti-CD44. The red histogram represents control cells and the blue histogram represents the cells incubated with the indicated antibodies. Cells cultured according to protocol 2 were positive for CD45, CD31, CD11b, Sca1 and CD44 and negative for Ter119. (D) Cells cultured under protocol 3 after 7 days in culture. Cells presented either an elongated shape (arrows) and were spread in the flask or exhibited a dark rounded shape organized in clusters. (E) Expression of the differentiation markers was similar to protocol 2. (F) Cells after 7 days of culture performed according to protocol 4, showing adherent cells with a slightly elongated shape (arrows) coexisting in the same flask with non-adherent cells (shadows of the cells floating in the media). (G) Cells cultured following this protocol were positive for all surface markers. (H) Cells cultured according to protocol 5 after 25 days of plating, showing a few small round cells spread in the flask and some spindle-shaped cells (arrows) organized in clusters and forming a monolayer (phase contrast image). (I) Similar to protocols 2 and 3, cells cultured according to protocol 5 were also positive for CD31, CD11b, Sca1 and CD44 and CD45 and negative for Ter119.
Characteristics of murine BM-MSC cultures obtained with the tested protocols.
FBS: Fetal Bovine Serum; HS: horse serum.
Protocol 2 generated fibroblast-like cells observed on the day of adherent cell selection in 10% and 20% HS conditions. However, on the 10th day, more elongated cells could be observed in the 20% HS condition, whereas unviable cells (round and dark under light microscopy) were observed in the 10% HS condition (Figure 1B). Cells cultured with 20% HS reached approximately 80% confluency and were sub-cultured for the first time after 22 days, whereas the cells cultured with 10% HS cells reached the necessary confluency to be first sub-cultured after 35 days (passage 1). Purity was analyzed on the 56th day (passage 2), when both conditions (10% and 20% HS) reached approximately 80% confluency and showed positive expression of CD45, CD31, CD11b and CD44, a low expression of Sca1 and a lack of expression of Ter119 (Figure 1C)(Table 2).
Cells cultured according to protocol 3 presented similar morphology to the cells cultured according to protocol 2 with 20% HS (Figure 1D). The addition of L-glutamine and hydrocortisone presented no apparent effects in the growth pattern. Purity was analyzed after 35 days of culture, when the cultures had not yet reached the necessary confluency to be sub-cultured (Table 2). Similar to protocol 2, these cells showed the expression of CD45, CD31, CD11b and CD44, a low expression of Sca1 and a lack of expression of Ter119 (Figure 1E).
Cultures based on protocol 4 (initial density of 31.9 × 107cells/175cm2) grew faster, reaching 80% confluency in 7 days, whereas the cells cultured according to protocol 5 (initial density of 8 × 107cells/175 cm2) reached the same confluency only after 28 days. On the day of the first passage, cells cultured according to protocol 4 showed non-adherent cells coexisting with elongated adherent cells (Figure 1F) and then stopped growing, not reaching the necessary confluency to be sub-cultured. The purity analysis of these cells showed the expression of all tested markers (Ter119, CD45, CD31, CD11b, Sca1 and CD44) (Figure 1G).
Protocol 5 generated two different morphologies of adherent cells: small round cells spread in the flask and elongated cells organized in clusters (Figure 1H). These cells kept growing until passage 6 (Table 2), when we observed a similar profile of expression markers of protocols 2 and 3 (Figure 1I).
DiscussionSeveral techniques have been published in the attempt to isolate and expand pure murine BM-MSCs for in vitro studies. Those techniques include cell-sorting,19 negative and positive selection20 and low- and high-density cultures.21In this study, we tested five protocols based on the adhesion potential of BM-MSCs to the tissue plastic culture, varying the type of culture medium, supplementation and initial cell density. Our results showed that none of the tested protocols were effective in generating a cell culture with high purity and the proper growth rate.
There is not a unique definition for murine BM-MSCs, which increases the difficulty in isolating and characterizing these cells, however some minimum criteria have been used for their identification, according to the expression of specific surface markers and ability to differentiate in vitro.4,12 We adopted the murine BM-MSCs surface marker panel that requires the positive expression of CD44 and Sca1, which are respectively expressed in BM-MSCs from all species and in the C57Bl/6 strain, and the negative expression of Ter119 (erythroid-specific antigen), CD45 (hematopoietic cell marker), CD31 (endothelial cell marker) and CD11b (expressed in monocytes, neutrophils, peritoneal B-1 cells, CD8+ dendritic cells, NK cells and a subset of CD8+ T cells).13 This panel was useful for the identification of the main cell types that could be present as contaminants. Our results showed that initial cell density seems to be a major factor in the growth rate, since cells cultured following protocol 4 (initial cell density of 31.9 × 107cells/175cm2) reached 80% of confluency in 7 days, whereas the cells cultured according to protocol 5 (initial density of 8 × 107cells/175 cm2) reached the same confluency in 28 days, despite the increase in serum concentration. However, the initial cell growth rate does not seem to be the only factor influencing the number of passages, as the cell culture based on protocol 4 stopped growing after the first passage, whereas cultures based on protocol 5 kept growing until the 6th passage.
The main reasons for terminating the cultures were the detection of hematopoietic cells (CD45+ and CD11b+), a very slow growth rate, or both. Depletion of the hematopoietic cells by positive and negative selection,20 sorting BM-MSCs by FACS21 or frequent medium change in primary culture and reduced trypsinization22 may be further included to decrease the presence of these contaminants. Since the BM-MSCs cultures were not free of other cell types, we did not attempt to freeze cells or test their capacity of differentiation.
Cultures based on protocol 5 presented a satisfactory growth rate and MSC-like cell morphology (spindle-shaped cells), however, the cells did not show the required purity. This is in accordance with Prockop et al.,23 who suggested that, in some cases, the few surviving cells undergo events presented as “multistage carcinogenesis in culture”, suffering a “crisis” and grow rapidly after acquiring spontaneous mutations.23–25 Those mutations may lead to different phenotypes, resulting in non-reproducible experiments and cells that do not fit into the mesenchymal cell marker panel.23
Boregowda et al.26 observed that the exposure of murine BM-MSCs to standard atmospheric oxygen conditions rapidly induces the expression of p53, TOP2A and BAX and increases the production of mitochondrial ROS, resulting in oxidative stress, reduced cell viability and inhibition of cell proliferation. In contrast, the long-term culture of BM-MSCs selects cells with impaired p53, preventing apoptosis. Rodent BM-MSCs were maintained in an undifferentiated state when kept in a low oxygen environment, whereas human BM-MSCs thrive.24,27–30 Therefore, murine BM-MSCs seem to be very sensitive to the oxygen concentration.
Our results indicated that additional approaches must be taken into consideration when culturing murine BM-MSCs. Among the tested protocols, we consider that protocol 5 presented the best performance, regarding the cellular growth rate and morphology (spindle-shaped appearance). Thus, we believe that steps, such as positive selection, depletion of macrophages and variation of oxygen concentration, may be included in this protocol to better succeed in obtaining pure murine BM-MSCs with a satisfactory growth rate.
ConclusionThe importance of this study lies in enlightening researchers on the problems associated with the current techniques used to culture murine BM-MSCs. Our results may guide the improvement of these protocols for achieving a pure culture of murine BM-MSCs for diverse applications.
FundingThis study was supported by São Paulo Research Foundation (FAPESP - grants 2017/19674-2, 2017/21801-2), National Council for Scientific and Technological Development (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brasil (CAPES) - Finance Code 001.
Author contributionsMariana Ferreira Pissarra performed experiments. Mariana Ferreira Pissarra, Cristina Okuda Torello and Mariana Lazarini analyzed the data. Mariana Ferreira Pissarra, Sara Teresinha Olalla Saad and Mariana Lazarini planned the study and prepared the manuscript. All authors read and approved the final manuscript.