Ruxolitinib has been approved for the treatment of myelofibrosis (MF). In this study, we present safety and efficacy findings from an analysis of 104 patients with intermediate- and high-risk MF in a Brazilian cohort of the JUMP study who received treatment with ruxolitinib.
MethodsJUMP is a single-arm, open-label, phase IIIb, expanded-access study. The primary endpoint was to evaluate the safety and tolerability (frequency, duration, and severity of adverse events [AEs]) of ruxolitinib.
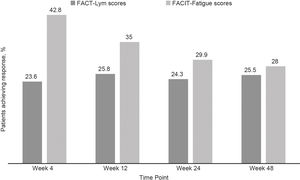
ResultsAll of the 104 patients received the treatment. Median duration of exposure was 35.8 months. The most common hematologic AEs were anemia (57.7), thrombocytopenia (38.5%), neutropenia (11.5%), and leukopenia (9.6%). Second malignancies (all grades) occurred in 19.2% of patients (n=20). Serious AEs were reported in 62.5% of patients (n=65). The proportions of patients with ≥50% reduction from baseline in palpable spleen length at weeks 24 and 48 were 62.7% and 69.2%, respectively. The mean change from the baseline in the Functional Assessment of Cancer Therapy (FACT)-Lymphoma total score was 10.8 [15.6%] at week 4, 12.6 [14.1%] at week 24, and 12.2 [14.3%] at week 48. The mean change from the baseline for the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale was 3.9 [42.8%] at week 4, 4.9 [29.9%] at week 24, and 4.7 [28%] at week 48. At week 48, the estimated progression-free survival, leukemia-free survival, and overall survival probabilities were 91%, 91% and 93%, respectively Overall, 21 deaths were observed in the present study.
ConclusionFindings from this study suggest that ruxolitinib could be evaluated as a standard-of-care treatment for the MF population in need of a viable treatment option. NCT01493414
Myelofibrosis (MF) is a rare, chronic, Philadelphia chromosome – negative myeloproliferative neoplasm (MPN).1 The pathophysiology of this disorder is the proliferation of clonal hematopoietic stem cells,2 which leads to an increase in bone marrow fibrosis, stromal changes, involvement of extramedullary organs such as the spleen and the liver, and consequent clinical manifestations.3 The disease presentation, however, is heterogeneous, with 30% of patients being initially asymptomatic.1 This variation in clinical phenotype warrants careful risk stratification to guide appropriate management, and prognostic risk scores are being continually refined.1
Based on the World Health Organization (WHO) 2016 criteria, 50%–60% of MF patients are carriers of the JAK2 V617F mutation.4–7 Among JAK2 inhibitors, ruxolitinib has been approved for the treatment of intermediate- and high-risk MF.8,9 In previous trials (including the COMFORT trials), ruxolitinib treatment led to durable improvements in splenomegaly, symptoms, and quality-of-life (QoL) measures, as well as improved survival.8–11 JUMP, a phase IIIb, expanded-access trial for patients without access to ruxolitinib outside of a clinical study, is the largest clinical trial to date on patients with MF who have been treated with ruxolitinib.11 Safety and efficacy findings from an analysis of 2233 patients with intermediate- or high-risk MF,11 as well as a separate analysis of 163 patients with intermediate-1–risk MF12 (a patient population that was not included in the phase III COMFORT studies), have been previously published.
Published literature identified the JAK2 V617F mutation to be prevalent in patients with MPN in Brazil. A study conducted on Brazilian patients with MPN (c.1887G>T) detected JAK2 V617F mutations in 56% of MF patients.13 Another Brazilian survey reflected a scarcity of diagnostic tests for MF patients.14 A 2013 study on patterns and costs associated with MF in Brazil reported the use of chemotherapy as a predominant treatment option.15 These studies highlight the lacunae in addressing the treatment needs of patients with MF in Brazil and the necessity to evaluate the role of ruxolitinib as a standard therapy option in this cohort.14
Here, we present safety and efficacy findings from an analysis of 104 patients with intermediate- and high-risk MF in a Brazilian cohort.
MethodsEligibility criteriaPatients aged ≥18 years who met the WHO recommended criteria for the diagnosis of primary MF and MF secondary to essential thrombocythemia (ET) and polycythemia vera (PV) were eligible for the current study.16 Eligible patients had intermediate-2- or high-risk MF by the International Prognostic Scoring System (IPSS) criteria, with or without splenomegaly, or IPSS intermediate-1-risk MF with a palpable spleen (≥5cm from the costal margin) (information collected after protocol amendment 2). The IPSS risk status stratification was included per protocol amendment. Patients were included if they had a baseline platelet (PLT) count of ≥50×109/L; patients with PLT counts of 50–100×109/L were enrolled per amendments. Informed consent was obtained from all the patients.
Study designJUMP is a single-arm, open-label, phase IIIb, expanded-access study. The starting doses of ruxolitinib were based on the PLT counts: 5mg twice daily (bid; 50–<100×109/L), 15mg bid (100–200×109/L), or 20mg bid (>200×109/L). Dose titration was allowed (per protocol) during treatment for safety and efficacy reasons, so that each patient was titrated to their most appropriate doses (5-mg-bid increments, up to a maximum of 25mg bid). Patients were followed for 28 days after the end-of-treatment visit (no data were collected for patients beyond the follow-up visit, including for those who transitioned to receive commercial ruxolitinib).
EndpointsThe primary endpoint was to evaluate the safety and tolerability of ruxolitinib, which included the frequency, duration, and severity of adverse events (AEs). Additional endpoints included the proportion of patients with ≥50% reduction in palpable spleen length (assessed by the International Working Group-Myeloproliferative Neoplasms Research and Treatment [IWG-MRT] criteria; Supplementary data), patient-reported outcomes (Functional Assessment of Cancer Therapy–Lymphoma [FACT-Lym] total score [TS] and Functional Assessment of Chronic Illness Therapy [FACIT]–Fatigue scores), progression-free survival (PFS), survival without transformation to leukemia (leukemia-free survival [LFS]), and overall survival (OS). Statistical analysis is discussed under Supplementary data.
ResultsPatientsA total of 2233 patients were enrolled at 279 clinical sites across 26 countries in the JUMP study (the last patient's last visit was on January 26, 2017). This subgroup analysis was conducted on 104 patients in the overall JUMP study cohort who were enrolled in Brazil. The trial was conducted in agreement with appropriate local regulations and the principles of the Declaration of Helsinki, and approved by the institutional review board at each participating institution. Written informed consent was collected from all the patients. All patients who received ruxolitinib were included in this analysis.
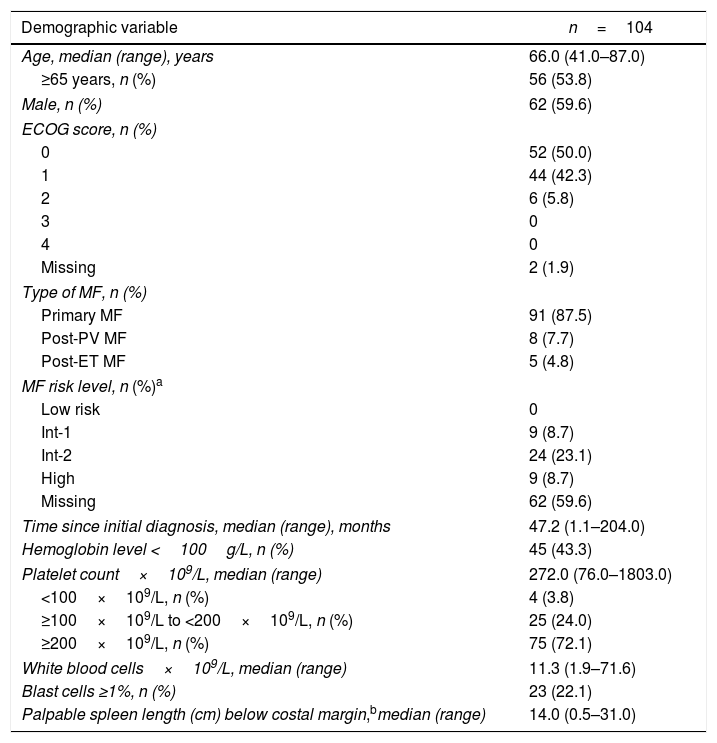
Baseline characteristics are presented in Table 1. The median age was 66 years (range, 41–87 years); 53.8% of the patients were ≥65 years. The median time from the initial diagnosis was 47.2 months (range, 1.1–204 months). Overall, 59.6% of patients were male, and 87.5% had primary MF.
Baseline characteristics.
| Demographic variable | n=104 |
|---|---|
| Age, median (range), years | 66.0 (41.0–87.0) |
| ≥65 years, n (%) | 56 (53.8) |
| Male, n (%) | 62 (59.6) |
| ECOG score, n (%) | |
| 0 | 52 (50.0) |
| 1 | 44 (42.3) |
| 2 | 6 (5.8) |
| 3 | 0 |
| 4 | 0 |
| Missing | 2 (1.9) |
| Type of MF, n (%) | |
| Primary MF | 91 (87.5) |
| Post-PV MF | 8 (7.7) |
| Post-ET MF | 5 (4.8) |
| MF risk level, n (%)a | |
| Low risk | 0 |
| Int-1 | 9 (8.7) |
| Int-2 | 24 (23.1) |
| High | 9 (8.7) |
| Missing | 62 (59.6) |
| Time since initial diagnosis, median (range), months | 47.2 (1.1–204.0) |
| Hemoglobin level <100g/L, n (%) | 45 (43.3) |
| Platelet count×109/L, median (range) | 272.0 (76.0–1803.0) |
| <100×109/L, n (%) | 4 (3.8) |
| ≥100×109/L to <200×109/L, n (%) | 25 (24.0) |
| ≥200×109/L, n (%) | 75 (72.1) |
| White blood cells×109/L, median (range) | 11.3 (1.9–71.6) |
| Blast cells ≥1%, n (%) | 23 (22.1) |
| Palpable spleen length (cm) below costal margin,bmedian (range) | 14.0 (0.5–31.0) |
n=patients with non-missing palpable spleen length at baseline.
Time since initial diagnosis=(treatment start date−date of MF disease first diagnosis)×12/365.25.
CRF: case report form; ECOG: Eastern Cooperative Oncology Group; ET: essential thrombocytopenia; Int: intermediate; MF: myelofibrosis; PV: polycythemia vera.
All of the 104 Brazilian patients received treatment. Overall, 43.3% of the patients completed the treatment duration as per protocol. The most common reasons for discontinuation included death (17.3%), AEs (16.3%), and disease progression (12.5%) (Table 1, Supplementary data).
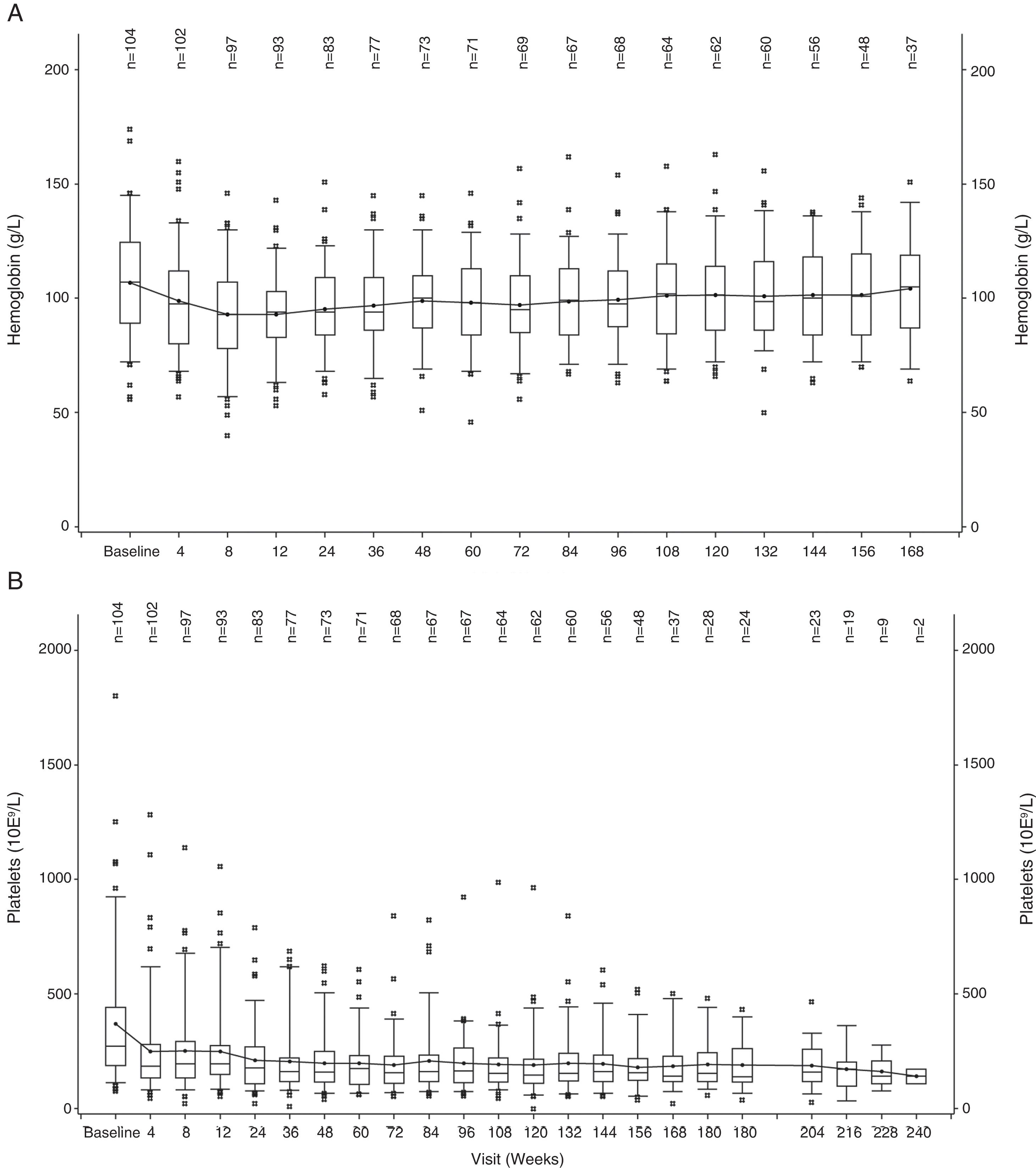
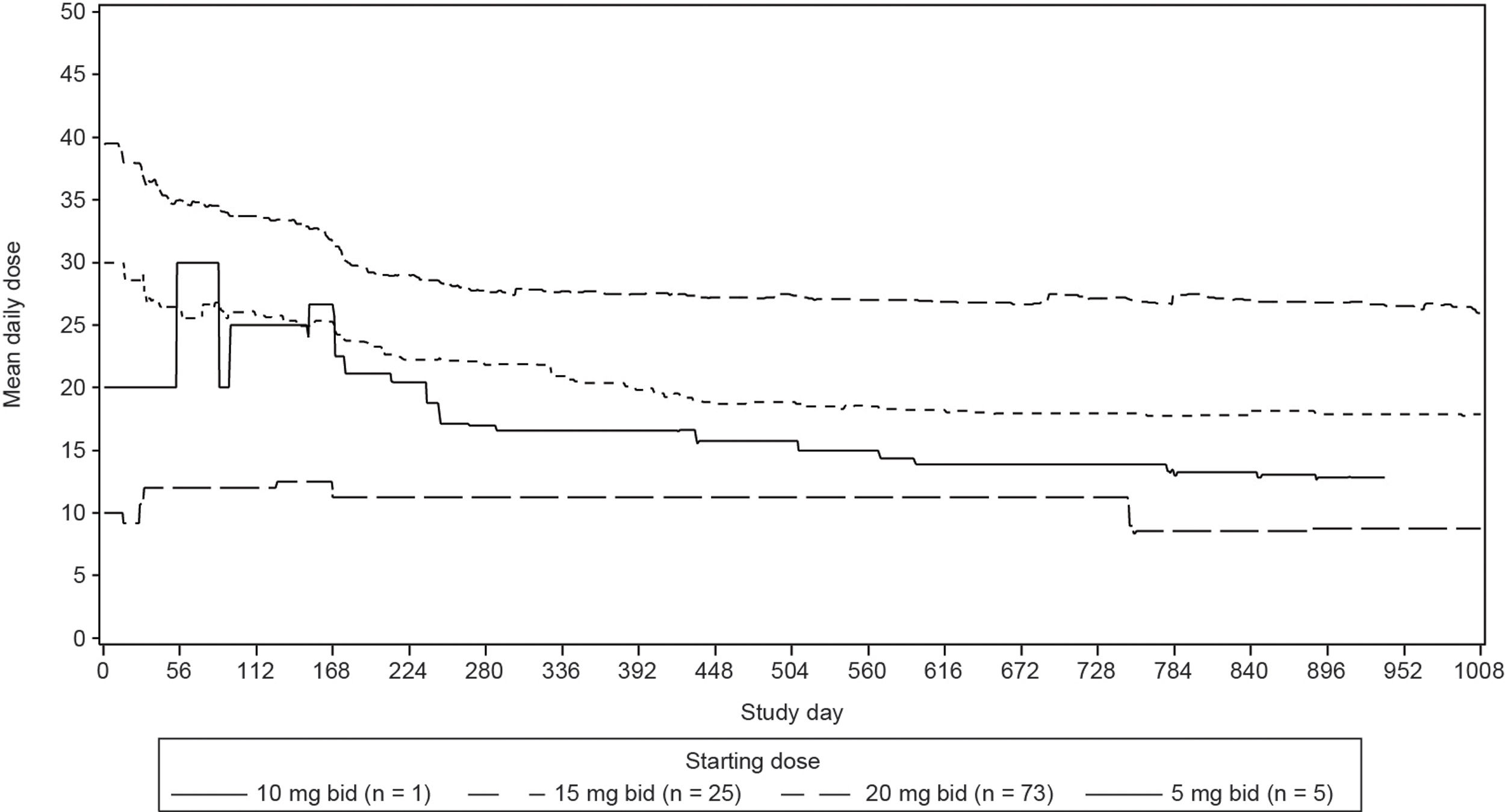
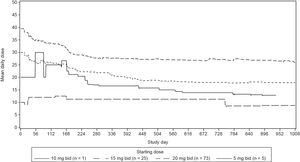
Median duration of exposure was 35.8 months (range, 0.6–59.7 months). Mean daily dose was 31.3mg for the Brazilian cohort (Figure 1, Supplementary data). The majority of the patients (69.2%) had at least one dose reduction/interruption, and the most common reason was AEs (54.8%).
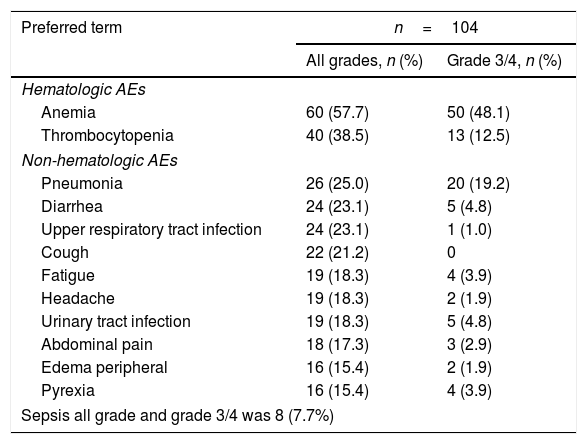
SafetyThe most common (in ≥5% of patients) hematologic AEs (Table 2) were anemia (all grades, 57.7%; grades 3/4, 48.1%), thrombocytopenia (all grades, 38.5%; grades 3/4, 12.5%), neutropenia (all grades, 11.5%; grades 3/4, 10.6%), and leukopenia (all grades, 9.6%; grades 3/4, 6.7%), and these results were consistent with the findings in the COMFORT studies.8–10 Of note, anemia (2.9%) was the only hematologic AE (in >1% of the patients) that led to study drug discontinuation (regardless of study drug relationship) in the Brazilian patient pool; no patient discontinued due to thrombocytopenia.
Frequent AEs regardless of study drug relationship (in ≥15% of patients).
| Preferred term | n=104 | |
|---|---|---|
| All grades, n (%) | Grade 3/4, n (%) | |
| Hematologic AEs | ||
| Anemia | 60 (57.7) | 50 (48.1) |
| Thrombocytopenia | 40 (38.5) | 13 (12.5) |
| Non-hematologic AEs | ||
| Pneumonia | 26 (25.0) | 20 (19.2) |
| Diarrhea | 24 (23.1) | 5 (4.8) |
| Upper respiratory tract infection | 24 (23.1) | 1 (1.0) |
| Cough | 22 (21.2) | 0 |
| Fatigue | 19 (18.3) | 4 (3.9) |
| Headache | 19 (18.3) | 2 (1.9) |
| Urinary tract infection | 19 (18.3) | 5 (4.8) |
| Abdominal pain | 18 (17.3) | 3 (2.9) |
| Edema peripheral | 16 (15.4) | 2 (1.9) |
| Pyrexia | 16 (15.4) | 4 (3.9) |
| Sepsis all grade and grade 3/4 was 8 (7.7%) | ||
Preferred terms are sorted by descending frequency, as reported in the “All grades” column. A patient with multiple occurrences of an AE is counted only once in the AE category. A patient with multiple AEs is counted only once in the total row. AEs occurring more than 28 days after the discontinuation of study treatment are not summarized.
AE: adverse event.
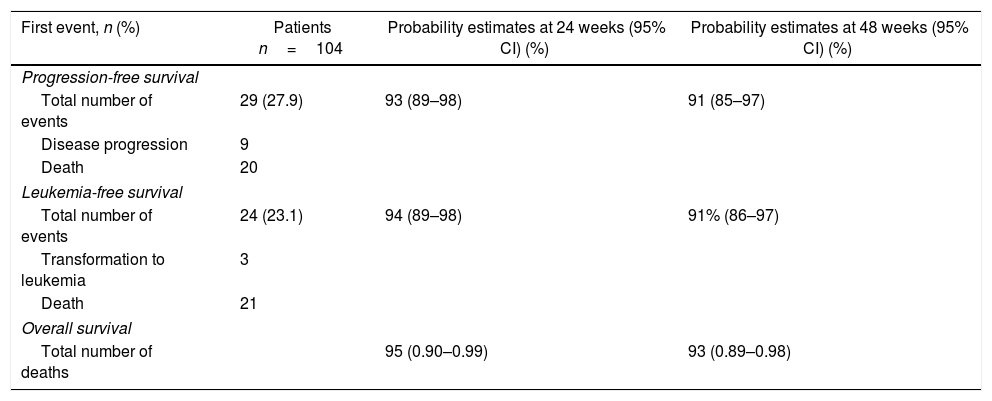
The hemoglobin level declined during the first 12 weeks of treatment, but increased to near-baseline levels after week 12 (Figure 1A). A similar trend was observed for PLT counts, wherein the values decreased during the first 4 weeks and then remained stable over time (Figure 1B).
The most common non-hematologic AEs (in ≥5% of the patients) were primarily grades 1/2 (Table 2), except pneumonia (19.2%, grades 3/4) and sepsis (7.7%, grades 3/4); no discontinuation was reported for these two non-hematologic AEs. Rates were low (<5%) for the other non-hematologic AEs. The rate of infections in all grades was 76.9%. Herpes zoster infection was reported in 9.6% of the patients (n=10; grade 3/4, 1% [n=1]), but no treatment discontinuation was reported for any of these patients. Tuberculosis was reported in one patient (1%) and this led to treatment discontinuation.
Second malignancies that occurred in 19.2% of patients (n=20) in all grades included squamous cell carcinoma (5.8% [n=6]), basal cell carcinoma (3.9% [n=4]), skin neoplasm (2.9% [n=3]), prostate cancer (1.9% [n=2]), and squamous cell carcinoma of the skin (1.9% [n=2]). Other second malignancies occurred in 1% of patients; there was one case each of acute leukemia, leukemia, B-cell lymphoma, benign parathyroid tumor, and skin papilloma. Acute myeloid leukemia (disease progression) occurred in two (1.9%) patients.
Serious AEs were reported in 62.5% of patients (n=65). Serious AEs occurring in >2% of patients in all grades included pneumonia (21.2%), sepsis (7.7%), urinary tract infection (4.8%), anemia (4.8%), septic shock (3.9%), squamous cell carcinoma (3.9%), respiratory failure (3.9%), ascites (3.9%), bronchospasm (2.9%), skin infection (2.9%), cardiogenic shock (2.9%), abdominal pain (2.9%), fatigue (2.9%), renal failure (2.9%), and dyspnea (2.9%) (Table 2, Supplementary data).
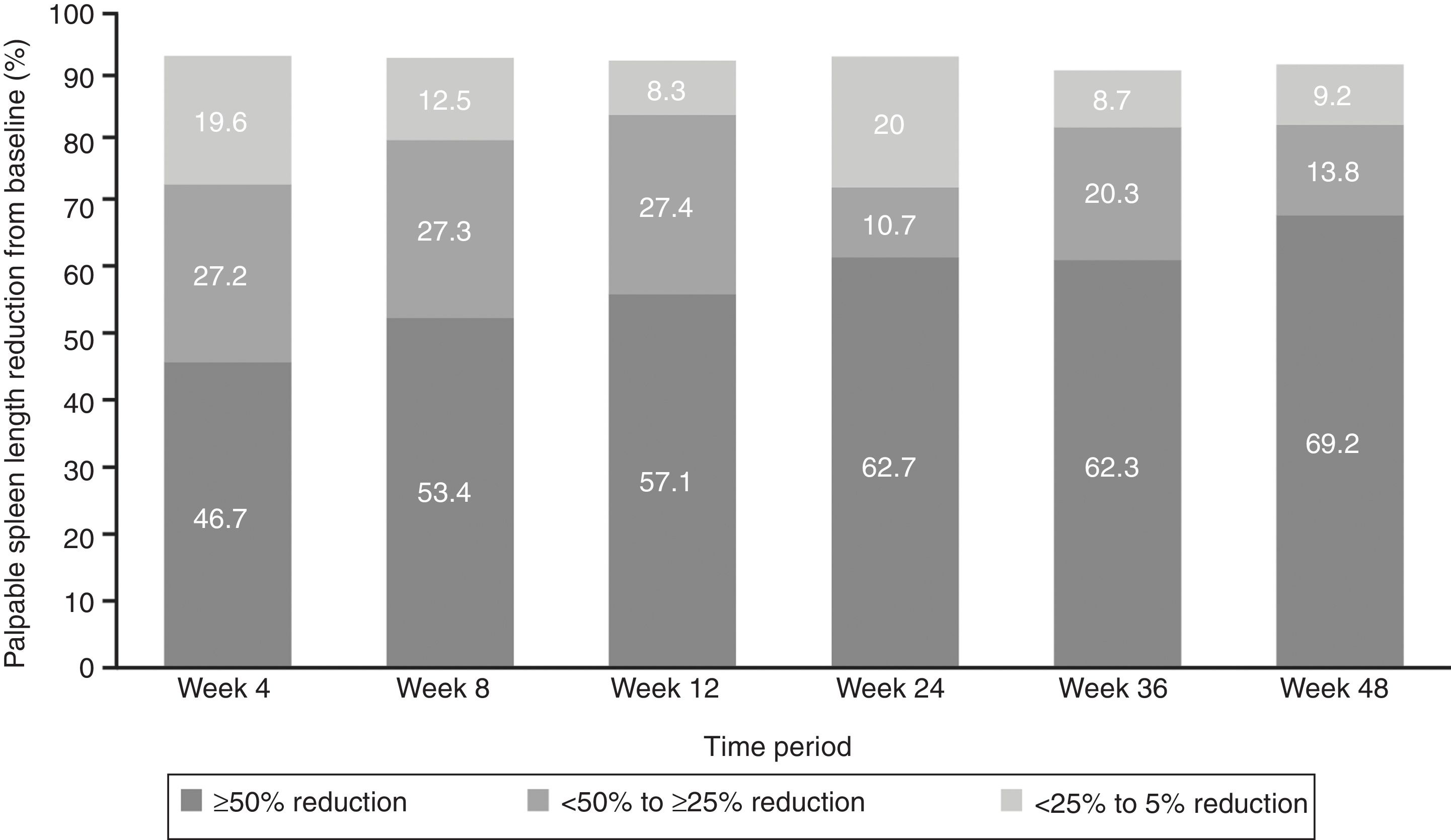
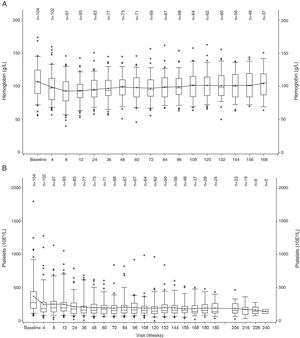
EfficacyThe proportion of patients with ≥50% reduction from baseline in palpable spleen length at weeks 24 and 48 was 62.7% and 69.2%, respectively, while 10.7% of patients at week 24 and 13.8% at week 48 showed 25% to <50% reductions (Figure 2).
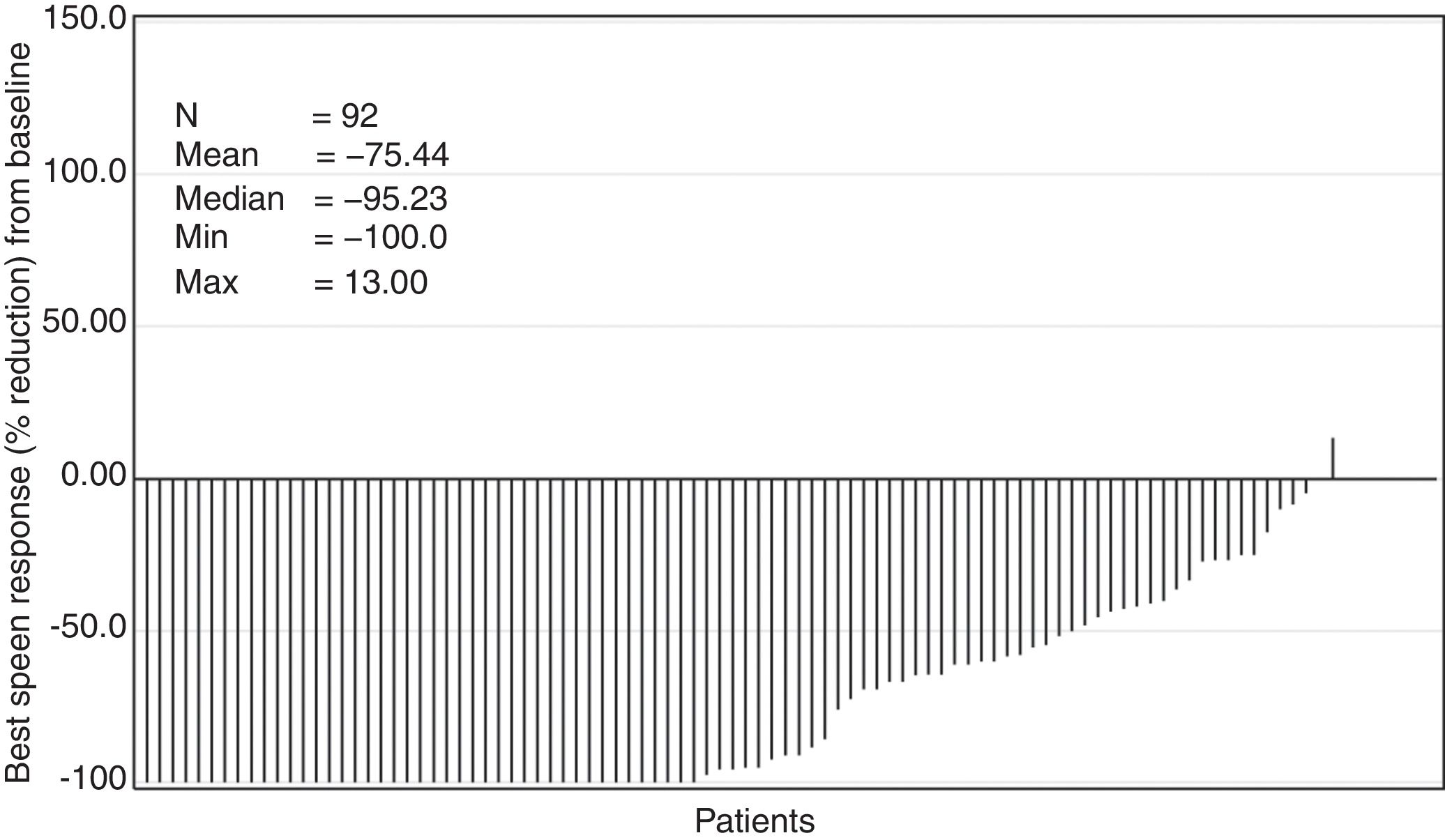
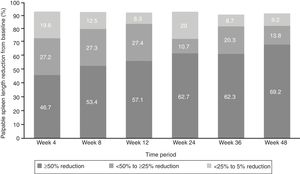
The median time to first ≥50% reduction in palpable spleen length was 4.3 weeks (range, 2.9–160.3 weeks). Overall, 78.3% of the patients showed ≥50% reduction in spleen length at any point in time in the study (Figure 3). The Kaplan–Meier estimate for duration of first response (≥50% reduction in spleen length from baseline) at 24 weeks and 48 weeks was 89% (95% confidence interval [CI], 78%–94%) and 86% (95% CI, 74%–92%), respectively.
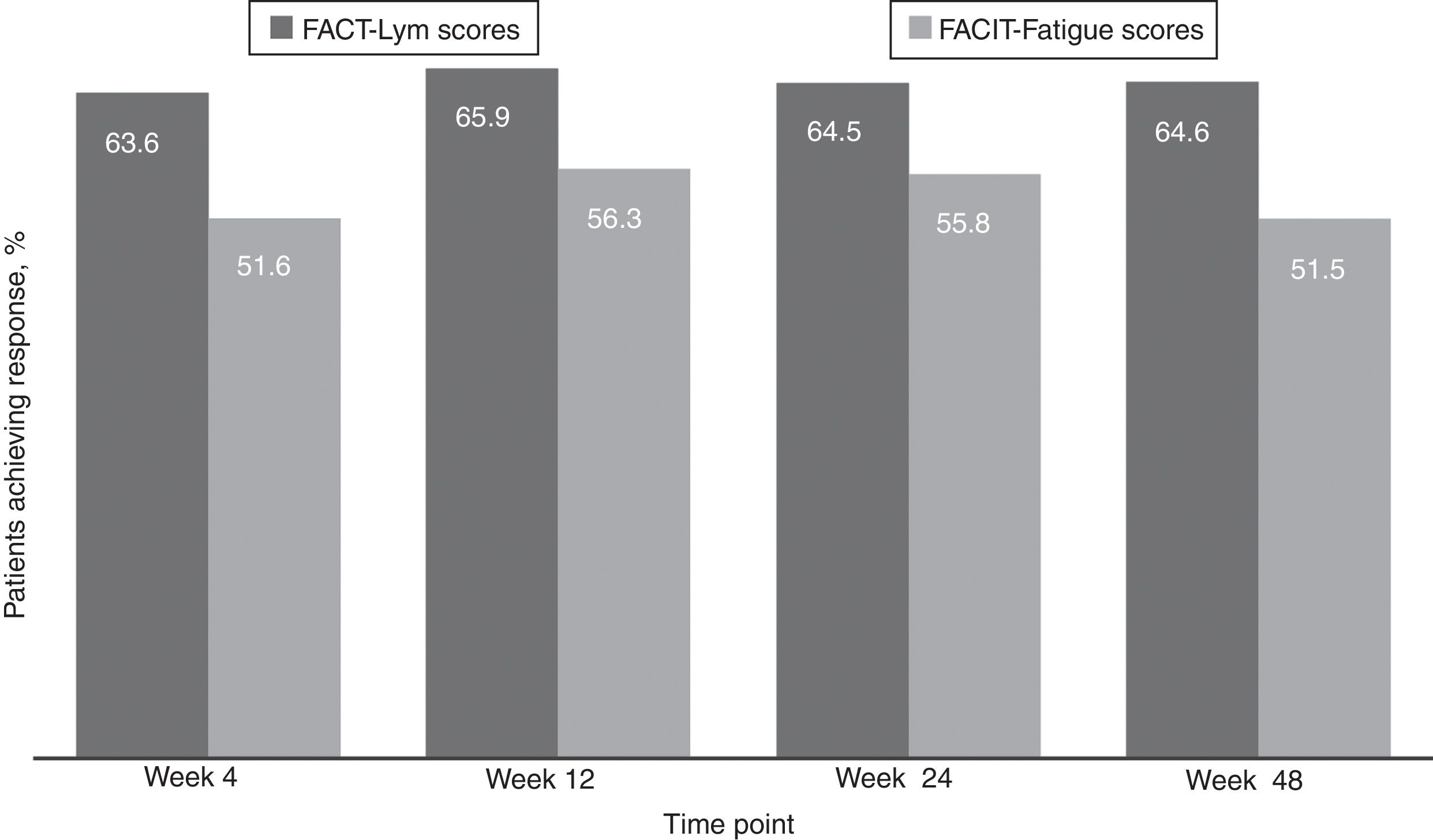
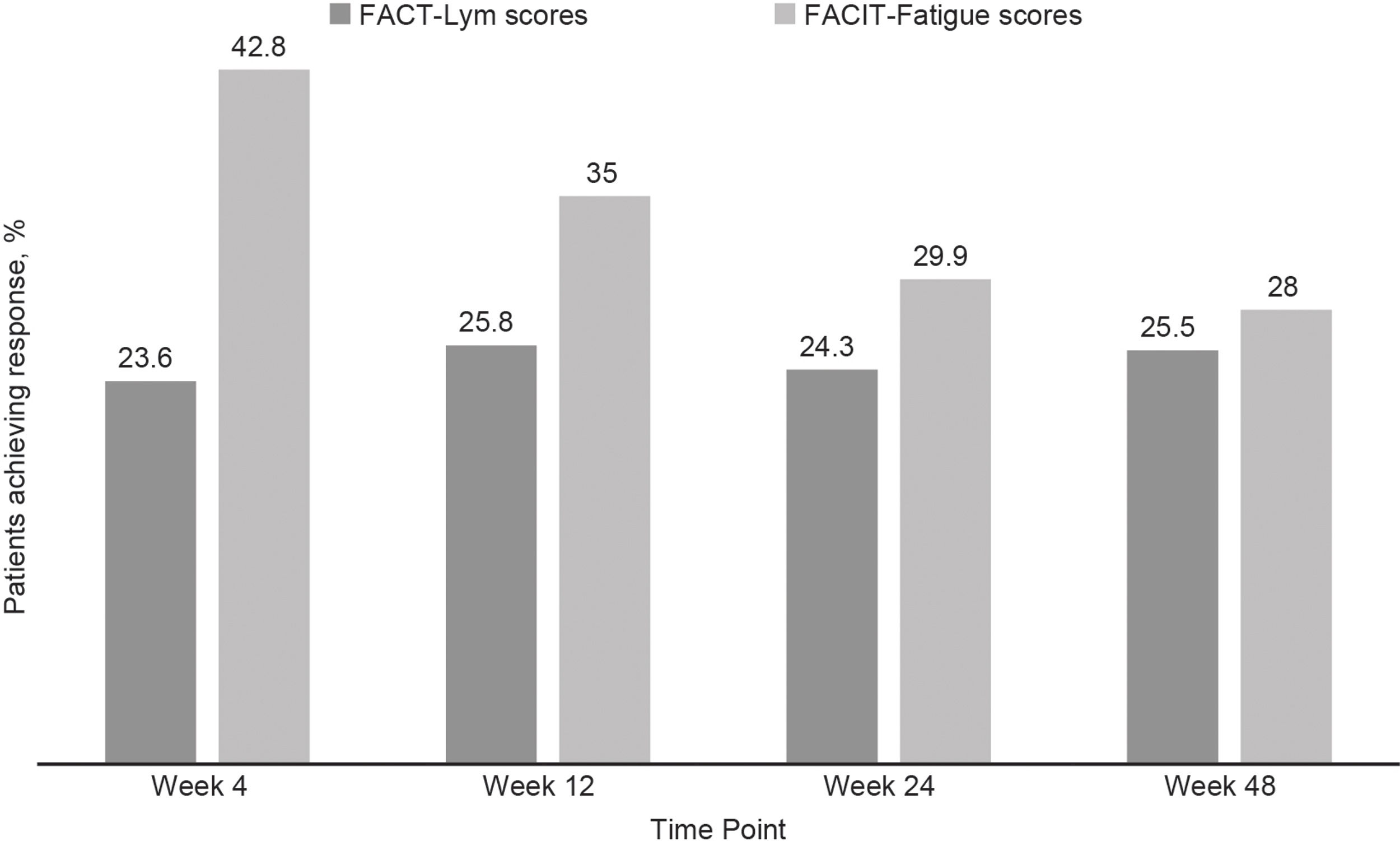
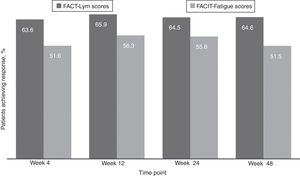
Clinically meaningful improvements in symptoms were seen as early as 4 weeks after the treatment initiation and were maintained over time, as evaluated by the FACT-Lym TS (mean change from baseline was 10.8 [15.6%] at week 4, 12.6 [14.1%] at week 24, and 12.2 [14.3%] at week 48) and the FACIT-Fatigue scale (the mean change from baseline was 3.9 [42.8%] at week 4, 4.9 [29.9%] at week 24, and 4.7 [28%] at week 48) (Figure 2, Supplementary data).17,18 Overall, 64.5% and 64.6% of the patients achieved a response (health-related QoL [HRQoL]; i.e., minimally important difference) in the FACT-Lym TS and 55.8% and 51.5% in the FACIT-Fatigue scale at 24 and 48 weeks, respectively (Figure 4).
The estimated PFS probability at 48 weeks was 91% (95% CI, 85%–97%). The MF transformed into leukemia in three patients during the study. The estimated LFS probability at 48 weeks was 91% (95% CI, 86%–97%). The estimated OS probability at 48 weeks was 93% (95% CI, 89%–98%) for the Brazilian patient pool (Table 3).
Progression-free, leukemia-free, and overall survival estimates.
| First event, n (%) | Patients n=104 | Probability estimates at 24 weeks (95% CI) (%) | Probability estimates at 48 weeks (95% CI) (%) |
|---|---|---|---|
| Progression-free survival | |||
| Total number of events | 29 (27.9) | 93 (89–98) | 91 (85–97) |
| Disease progression | 9 | ||
| Death | 20 | ||
| Leukemia-free survival | |||
| Total number of events | 24 (23.1) | 94 (89–98) | 91% (86–97) |
| Transformation to leukemia | 3 | ||
| Death | 21 | ||
| Overall survival | |||
| Total number of deaths | 95 (0.90–0.99) | 93 (0.89–0.98) | |
CI: confidence interval.
Investigator-determined causes of death included MF (n=2), cardiac arrest (n=2), cardiogenic shock (n=2), pneumonia (n=2), sepsis (n=2), respiratory failure (n=2), and pulmonary embolism (n=2). All other causes were undetermined or reported for one patient each.
DiscussionA previous study performed on Brazilian patients with MF found that the JAK2 V617F mutation (c.1887G>T) was in accordance with previous screenings of this mutation in other populations.13 Another Brazilian population survey suggested a lack of awareness in Brazilian MF patients because a substantial number of medical reports do not provide a complete history, and 20%–30% of them do not have all the laboratory tools for a precise diagnosis of MF.15 This is a first-of-its-kind study evaluating the safety and efficacy of ruxolitinib in patients with MF in Brazil.
Ruxolitinib has been evaluated in patients with MF in previous studies. This report summarizes a subgroup analysis (Brazilian cohort) of the JUMP study.8–12
As expected, anemia and thrombocytopenia were the most common AEs, owing to the mechanism of action of ruxolitinib. The rate of discontinuation was low, indicating that hematologic side effects were well managed in this patient population.
Findings from previous studies reinforce the concept that disease severity could be a risk factor for infections in MF patients.19 A prior study suggested that IPSS scores and patient history were directly related to the risk of infections in MF patients during ruxolitinib therapy.20 In the Brazilian population, the number of patients aged >65 years with intermediate-2- and high-risk MF was high, and this could be a predisposing factor for infections.
Efficacy data were consistent with previous studies of ruxolitinib (COMFORT and JUMP).8–11 A slightly larger number of patients, particularly in the Brazilian cohort, had achieved ≥50% reduction from baseline in palpable spleen length versus patients in the overall JUMP cohort at week 24 (62.7% vs. 56.5%, respectively).11 Additionally, the median time to first ≥50% reduction in spleen length was lower (4.3 weeks) compared to the overall JUMP population (5.8 weeks).11 Although differences in study designs and patient populations prevent a direct comparison, these findings are similar to those in the phase III COMFORT studies, where patients treated with ruxolitinib experienced substantial reductions in spleen volume and improvements in symptoms.8–10
Ruxolitinib treatment showed a significant improvement in HRQoL in the Brazilian cohort, and this improvement was slightly higher, compared to that in the overall JUMP population. Our results were in concordance with a previous trial where ruxolitinib showed significantly higher response rates in global health status/QoL and FACT-Lym summary scores. This benefit offers better functional outcomes and a more normal day-to-day life with improved symptoms, and may strengthen MF patients to withstand a challenging, severely debilitating disease.21 Ruxolitinib also led to prolonged OS benefits (93% at week 48), which was the same as the results in the overall JUMP population (93% at week 48).11 Furthermore, the COMFORT pool OS analysis reported a reduction in death by 30% among patients randomized to ruxolitinib, compared with patients in the control group (median OS, 5.3 vs. 3.8 years, respectively; hazard ratio, 0.70 [95% CI, 0.54–0.91]; p-value=0.0065).22
Results of the Brazilian cohort were similar to those in the overall JUMP and COMFORT populations despite differences in ethnicity and socioeconomic factors, suggesting that these factors did not impact the safety or effectiveness of ruxolitinib in MF patients. In this study, ruxolitinib provided a manageable safety profile and clinically meaningful reductions in spleen size and symptoms along with a significant improvement in HRQoL and OS, consistent with the phase III COMFORT studies and the JUMP study. Findings from this study suggest that ruxolitinib could be evaluated as a standard-of-care treatment option for the MF population, for whom a viable standard treatment option is lacking.
Meeting of ethical standardsThe trial was conducted in agreement with appropriate local regulations and the principles of the Declaration of Helsinki, and approved by the institutional review board at each participating institution. Written informed consent was collected from all the patients.
FundingThis study was funded by Novartis.
Author contributionsAll authors contributed equally. All authors approved the manuscript.
Conflicts of interestRenato Tavares: No conflict of interest to declare.
Carmino De Souza: No conflict of interest to declare.
Ricardo Pasquini: No conflict of interest to declare.
Carole Paley: Employee of Novartis.
Catherine Bouard: Employee of Novartis.
Ranjan Tiwari: Employee of Novartis.
The authors would like to thank the following study investigators: Nelma Clementino, Laura Fogliatto, Carlos Chiattone, Nelson Hamerschlak, Carla Boquimpani, Belinda Simões, Mônika Conchon, Laura Fogliatto. The authors also thank Saurabh Agarwal for assistance in medical writing.