Acute lymphoblastic leukemia (ALL) is the cancer with the highest incidence in childhood and adolescence, and pharmacotherapy is the primary form of treatment.
Objective and methodsA systematic review of the efficacy and safety of polyethylene glycol (PEG)-asparaginase in acute lymphoblastic leukemia therapy in children and adolescents was conducted to compare it with native Escherichia coli L-asparaginase. PubMed, Web of Science, Science Direct, Cochrane Library, Scopus, LILACS (Latin American and Caribbean Health Sciences Literature) and EMBASE databases were selected. The following outcomes were analyzed: complete remission of the disease, event-free survival, overall survival, anti-asparaginase antibody level, hypersensitivity reactions, asparaginase and asparagine serum levels, number of postdiagnosis events, and overall mortality. Five randomized controlled trials were included. Analysis of the quality of evidence and risk of bias was performed using the Cochrane recommendation tool and the GRADE system.
ResultsThe assessment results suggest that the level of certainty on the technology addressed is relatively weak from a methodological point of view. Evidence is insufficient to assess the effects on health outcomes because of the limited number and power of studies and important flaws in their design or conduct in classifying PEG-asparaginase as a superior drug or not, in the pharmacotherapy of ALL in children and adolescents. PEG-asparaginase can be used as a substitute for native E. coli L-asparaginase, demonstrating similar efficacy and safety.
ConclusionThe study may help decision-makers in the public health system to offer a more in-depth judgment on the therapeutic alternatives used to treat this neoplasm in children and adolescents.
Leukemia is the child-juvenile neoplasia that presents the highest frequency worldwide (from 25% to 35%).1 Acute lymphoblastic leukemia (ALL) is the most common subtype in children and adolescents.2 Its prognosis has improved significantly over the years, partly due to effective antineoplastic therapy and well-developed treatment protocols.3
Native Escherichia coli L-asparaginase is an enzyme used in the treatment of ALL associated with improvement in clinical outcomes,4 but may cause hypersensitivity reactions or be inactivated by a specific antibody formation. These aspects limit its use and represent the main factors responsible for the interruption of the treatment.5,6
Different measures are available to improve therapeutic results and reduce the undesirable enzyme effects, including the use of asparaginase extracted from strains of Erwinia bacteria (Erwinase) or PEG-asparaginase use, an enzyme conjugated to polyethylene glycol (PEG) molecules.5 PEG-asparaginase has a low incidence of hypersensitivity and more advantageous pharmacokinetics, compared to other agents.7 Given the native E. coli L-asparaginase side effects, Narta and Graham recommend PEG-asparaginase therapy from the beginning, not only after allergic reactions.4,8
Is the replacement of native E. coli L-asparaginase with PEG-asparaginase justified? A systematic review of the efficacy and safety of pharmacotherapy with PEG-asparaginase and native E. coli L-asparaginase in children and adolescents with ALL was conducted to answer this question.
MethodsThe systematic review was conducted according to the Cochrane Collaboration9 recommendations and Brazilian Ministry of Health10 Guidelines. This study is registered in the International Prospective Register of Systematic Reviews (PROSPERO) under number CRD42017072495.
The research included scientific literature aimed at assessing the efficacy and safety of PEG-asparaginase in the treatment of ALL in children and adolescents, compared to other enzyme formulations. PubMed, Web of Science, Science Direct, Cochrane Library, Scopus, LILACS (Latin American and Caribbean Health Sciences Literature), and EMBASE were the databases used. The descriptors and MesH terms (Medical Subject Heading) consulted included ‘Pegaspargase’, ‘Asparaginase’, and ‘Precursor cell lymphoblastic leukemia lymphoma,’ associated with their entry terms and combined with the Boolean operators AND and OR. The filter for the terms associated with “randomized controlled trials” recommended by the Ministry of Health10 was used in the PubMed database. Clinical Trials and Google Scholar databases were used to assess gray literature. The search included a manual assessment of bibliographic references of the studies comprised in the review. Two independent reviewers participated in the selection (C.V.M. and T.M.A.C.), and a third researcher was consulted during disagreements (G.B.G.M.).
The research did not employ limits on the publication period and included papers until March 2018. Several languages, including English, Portuguese, Spanish, French and German, were considered. The PICOT strategy recommended by Cochrane9 was used to define the eligibility criteria. The study population consisted of patients aged 0–19 years with ALL. PEG-asparaginase (with or without previous therapy with other enzyme formulations) was the intervention assessed. Native E. coli L-asparaginase was used for the comparison, and the following outcomes analyzed were as follows: (a) complete disease remission; (b) event-free survival; (c) overall survival; (d) absence of complete disease remission; (e) number of postdiagnosis events; (f) overall mortality; (g) level of production of anti-asparaginase antibodies; (h) hypersensitivity; (i) asparaginase serum levels and (j) asparagine serum levels. Randomized controlled clinical trials (RCTs) were the selected studies. This study evaluated the methodological quality and risk of bias of the trials using the Cochrane methodology.9,11 The GRADE system (Grading of Recommendations for Assessment, Development, and Evaluation) was also used to assess the level of evidence for some outcomes for which statistical analyses were performed.12 The following statistical analyses were performed using the RStudio program13: absence of complete remission of the disease, number of postdiagnosis events, overall mortality and hypersensitivity. Qualitative analyses of the other outcomes were performed.
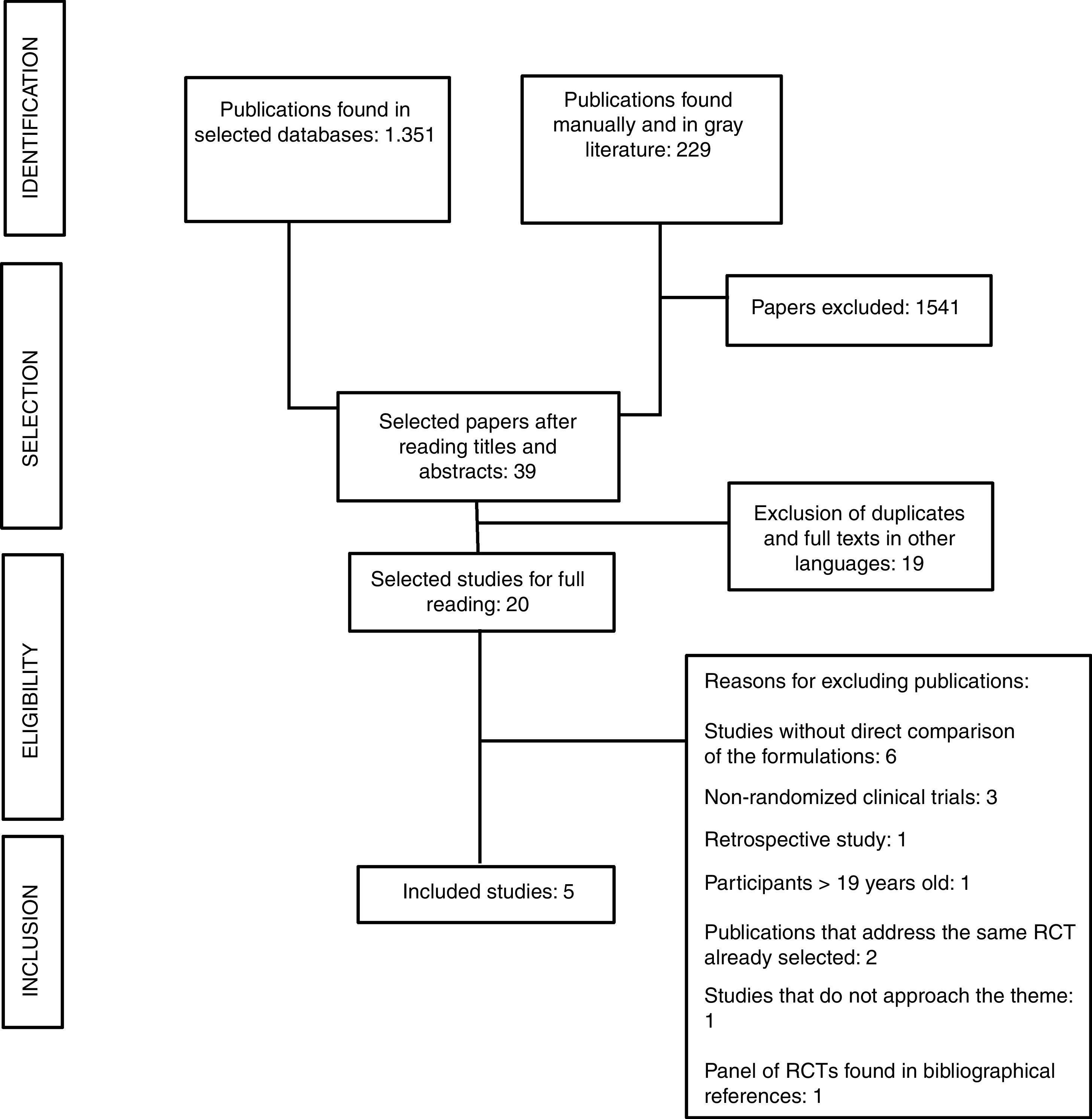
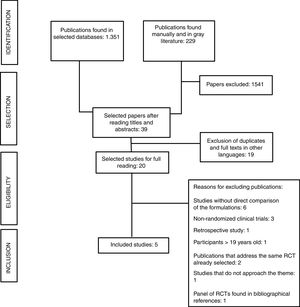
ResultsThe search procedure is described in the flowchart (Figure 1). Of the 1580 publications identified, 1541 did not address the research theme. After applying the eligibility criteria, five papers were selected for critical analysis. Figure 1 presents the search flowchart for the systematic review.
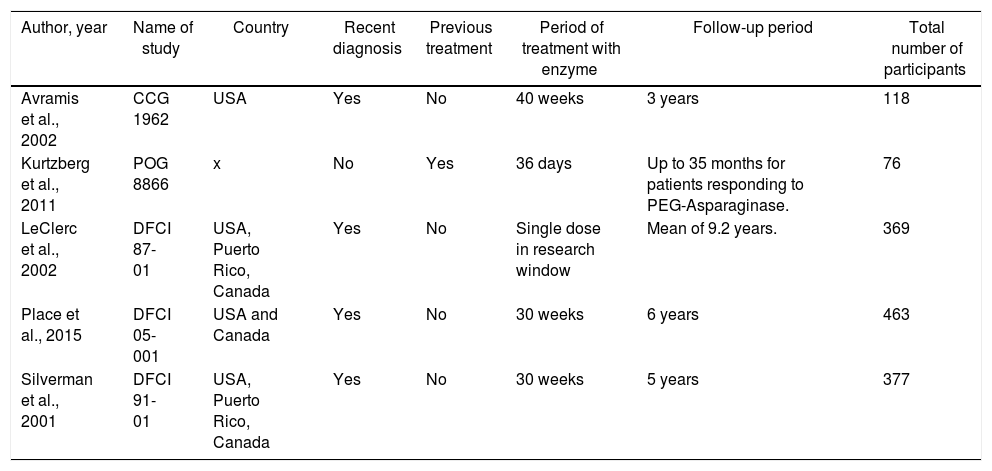
The CCG1962, POG 8866, DFCI 87-01, DFCI 05-001 and DFCI 91-01 trials were included.14–18 All five studies compared the two enzyme formulations; these trials evaluated the efficacy and safety aspects of the drug itself,14,15,17 or the therapy as a whole.16,18 Four studies used patients newly diagnosed with the disease and treatment-naïve patients: CCG 1962, DFCI 87-01, DFCI 05-001, and DFCI 91-01.14,16–18 One trial (POG 8866) recruited patients undergoing a second bone marrow relapse who had been previously treated with native E. coli L-asparaginase.15 This trial also recruited and analyzed the outcomes of patients with a history of allergy to native L-asparaginase; these patients were not randomized and were treated directly with PEG-asparaginase.15Table 1 presents the general data of the trials included in the review.
General data of selected studies.
| Author, year | Name of study | Country | Recent diagnosis | Previous treatment | Period of treatment with enzyme | Follow-up period | Total number of participants |
|---|---|---|---|---|---|---|---|
| Avramis et al., 2002 | CCG 1962 | USA | Yes | No | 40 weeks | 3 years | 118 |
| Kurtzberg et al., 2011 | POG 8866 | x | No | Yes | 36 days | Up to 35 months for patients responding to PEG-Asparaginase. | 76 |
| LeClerc et al., 2002 | DFCI 87-01 | USA, Puerto Rico, Canada | Yes | No | Single dose in research window | Mean of 9.2 years. | 369 |
| Place et al., 2015 | DFCI 05-001 | USA and Canada | Yes | No | 30 weeks | 6 years | 463 |
| Silverman et al., 2001 | DFCI 91-01 | USA, Puerto Rico, Canada | Yes | No | 30 weeks | 5 years | 377 |
In the subgroup analysis, the POG 8866 and DFCI 87-01 trials15,16 were highlighted in the heterogeneity assessment, as evidenced by the Inconsistency Test (I2) in the statistical analysis. The POG 8866 presented the lowest number of participants and the shortest treatment period compared to the other trials, except for the DFCI 87-01. The trial enrolled patients previously treated with native L-asparaginase and who were in a second bone marrow relapse.15 The DFCI 87-0116 was the only trial that randomized only one dose of each formulation and the remaining therapy included only native L-asparaginase. The other studies recruited over 100 individuals and presented a similar treatment period, and all patients were newly diagnosed and treatment-naïve.14,17,18
Complete disease remissionThe CCG 196214 and POG 886615 trials used the complete disease remission measure, defined as the presence of lymphoblasts less than or equal to 5% of the cells found in the bone marrow.14,15
In the CCG 196214 protocol, 52 patients (96% of the analyzed samples and 88% of the total randomized) achieved complete remission in the intervention group (PEG-asparaginase). In the control group (native L-asparaginase), 43 reached this result (83% of the analyzed samples and 73% of the total randomized). In the POG 8866,15 of the total number of patients taking PEG-asparaginase, including randomized and nonrandomized patients (59 subjects), 23 (38%) achieved complete remission, including 7 in the intervention group (41%). In the control group, 8 achieved this result (47%).
The statistical analysis of the two trials used data of individuals who did not achieve the expected result, namely, patients not exhibiting a complete remission. A higher number of patients who did not achieve complete remission were observed in the group of individuals treated with native E. coli L-asparaginase. The relative risk for the absence of remission was less than 1 in individuals who used PEG-asparaginase (0.68 and 0.72 in the fixed and random model analyses, respectively) with a significant difference between groups (p=0.05). The high heterogeneity between the studies (I2=73%) is related to the POG 8866, as reported in the subgroup analysis.
Event-free survivalThe CCG 196214 reported a 3-year event-free survival of 85% for the intervention group and 78% for the control group. The DFCI 87-0116 reported a 9.2-year event-free survival of 73% for PEG-asparaginase and 77% for native L-asparaginase. The DFCI 05-00117 reported a 5-year event-free survival of 90% for the intervention group and 89% for the control group. The DFCI 91-0118 also reported a 5-year event-free survival of 78% for the intervention group and 84% for the control group. This last trial used 106 individuals to calculate the outcome for PEG-asparaginase and 92 for native L-asparaginase. This number did not correspond to the total of subjects randomized.
As the trials used different periods for this outcome, the statistical analysis used an absolute measurement and calculated the number of postdiagnosis events. Postdiagnosis events are defined as any event of the following circumstances: failure of induction, death at induction, death during remission, disease relapse and secondary cancer.17 The number of events did not exhibit a significant difference between groups (p=0.53), with relative risk values of 1.04 and 1.05 for the fixed and random model analyses, respectively.
Overall survivalThe DFCI 87-01 and DFCI 05-00116,17 presented overall survival. In the first trial, the overall survival of 9.2 years with PEG-asparaginase was noted in 83% of patients, while the same for native L-asparaginase was 88%. In the DFCI 05-001 trial, the overall survival of 5 years was noted in 96% and 94%, respectively.
Statistical analysis calculated mortality from overall survival and observed no significant difference between the groups (p=0.13). The I2 was 56%, and methodological and clinical aspects of the DFCI 87-01 trial are related to this value.
Hypersensitivity reactionsThe CCG 196214 reported two (3%) hypersensitivity reactions in the intervention group and none in the control group. In the POG 8866,15 patients in the intervention group did not exhibit hypersensitivity reactions. In nonrandomized patients, 11 (26%) individuals reported reactions (seven of grade 1, two of grade 2 and two of grade 3). In the control group, 2 (11.7%) reactions were reported (both grade 3).
In the DFCI 05-001,17 the incidence of hypersensitivity was increased in the intervention group with 28 (12%) reactions (fourteen grades 1 and 2 reactions and fourteen grades 3 and 4 reactions); and 21 (9%) reactions were noted in the control group (thirteen grades 1 and 2 reactions and six grades 3 and 4 reactions).
The hypersensitivity reactions exhibited an increased tendency to occur in the intervention group, with a relative risk of approximately 1.2. However, the difference between the studies was not significant (p=0.32).
Anti-asparaginase antibody titersThe CCG 1962 trial14 classified the antibody titers as high when serum values were greater than 2.5. In the delayed intensification phase of the treatment, 26% of the patients in the control group exhibited increased antibody levels (11 of 43 children), whereas only 2% of the intervention group had high values (1 of 47) (p=0.0001). The POG 886615 classified values greater than 3 as high titers of antibodies. Low values were in the range of 0–2. For the intervention group, the values were 1.21± 1.48 at the baseline and 1.57±1.50 at the highest limit. For patients assigned directly to the PEG-asparaginase treatment, the values were 1.48±1.18 at the baseline and 2.45±1.06 at the highest limit. In the control group, the values presented were 0.25±0.62 for the baseline and 1.25±1.35 for the highest limit.
Serum levels of asparaginase and asparagineThe CCG 196214 measured asparaginase levels on day 21 of each delayed intensification phase. In the first delayed intensification, 95% of the patients in the intervention group had enzyme levels greater than 0.03IU/mL and 0.1IU/mL, defined as the minimum asparaginase level for depletion of asparagine to below 0.1μM3,19 and ideal level for enzymatic activity,14 respectively. In the control groups, 31% had levels greater than 0.03IU/mL, and 19% exhibited levels greater than 0.1IU/mL. In the second delayed intensification, 91% of the patients in the intervention group exhibited levels greater than 0.03IU/mL and 0.1IU/mL. In the control group, 39% of patients exhibited levels greater than 0.03IU/mL and 22% of the patients exhibited levels greater than 0.1IU/mL.
The POG 886615 reported the number of patients with enzyme levels greater than 0.0125IU/mL on days 7 and 14. In the intervention group, 83% (5/6) of the samples on day 7 were greater than this value, and 50% (3/6) of patient exhibited levels greater than this value on day 14. Among the nonrandomized patients, the values were 40% (4/10) and 20% (2/10), respectively. In the control group, the measurements were 100% (7/7) and 86% (6/7), respectively. Hypersensitive patients exhibited reduced serum levels of the enzyme.
The DFCI 05-00117 trial was measured at weeks 5, 11, 17, 23 and 29 of the treatment. The intervention group presented serum levels greater than 0.7IU/mL throughout the entire period. The control group presented values between 0.1IU/mL and 0.2IU/mL.
Only the CCG 196214 evaluated serum asparagine levels. Serum asparagine levels were less than 3μM in the majority of the patients with asparaginase levels greater than 0.1IU/mL.
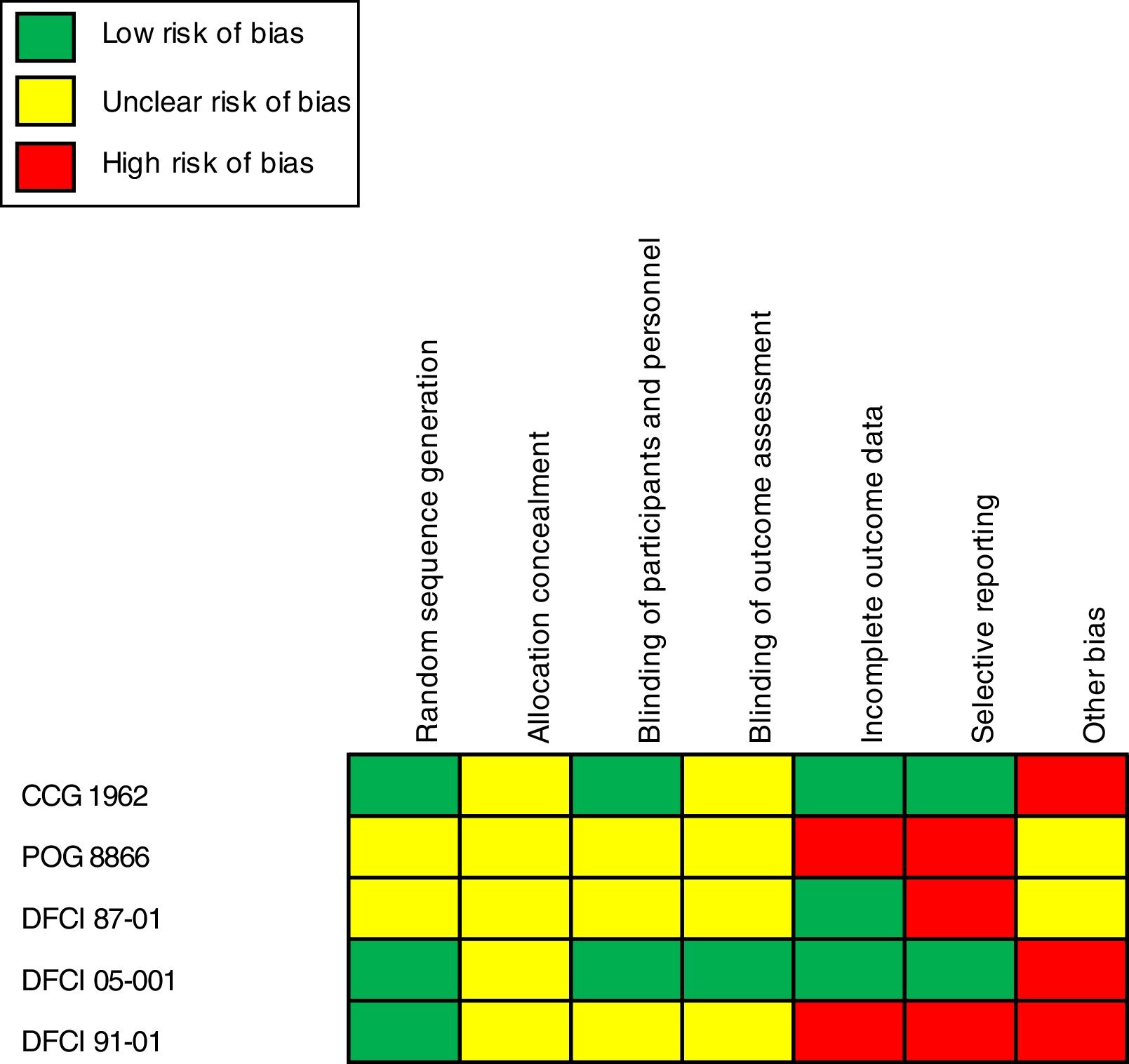
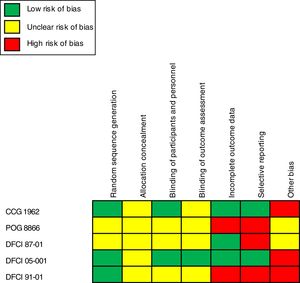
Risk assessment of bias and quality of evidenceApplication of the Cochrane recommendation toolThe general assessment of bias highlighted an essential extension of the unclear and high risk of bias associated with the lack of information in the trial methods. The category “another bias” exhibited a high risk of bias in two of five trials analyzed that were associated with pharmaceutical industry financing.14,17 The category “selective reporting” also reported a considerable degree of high risk of bias associated with a lack of detail for some of the outcomes reported and the inclusion of measures related to groups of nonrandomized patients.15,16,18Figure 2 presents the results on the risks of bias and quality of evidence after the insertion of data extracted from the trials in the Review Manager program.11
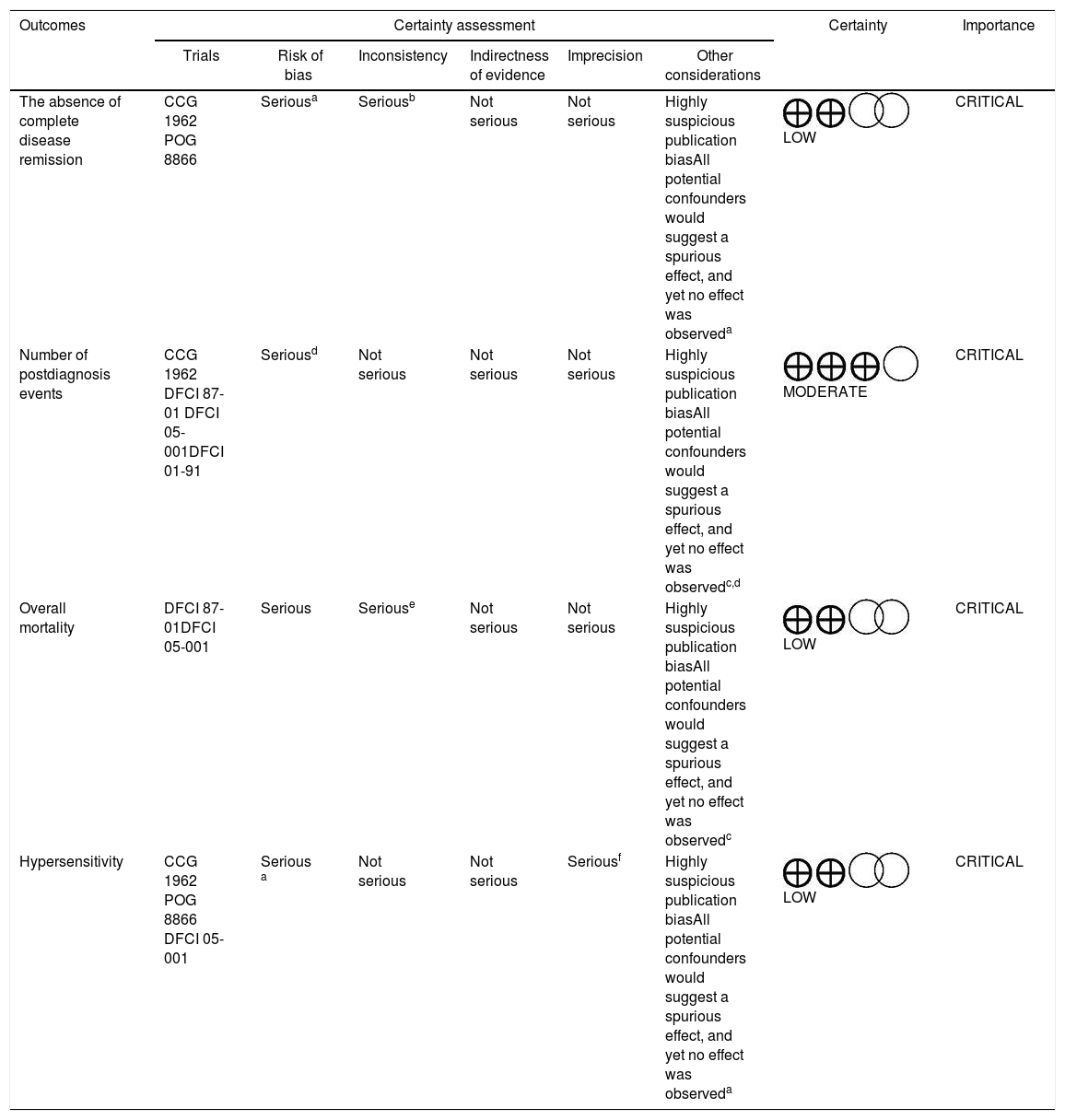
Application of the GRADE system in the evaluation of outcomesThe GRADE system12 was applied in the following outcomes: absence of complete disease remission, number of postdiagnosis events, overall mortality and hypersensitivity. Performing statistical analysis for these outcomes was possible.
The evidence evaluation process revealed that the absence of complete disease remission, overall mortality, and hypersensitivity presented a low level of evidence. The number of postdiagnosis events had a moderate level of evidence. Table 2 presents the analysis of these data. These results suggest that the power of recommendation on the technology addressed is relatively weak from a methodological point of view.
Certainty assessment of the outcomes – application of the GRADE system.
| Outcomes | Certainty assessment | Certainty | Importance | |||||
|---|---|---|---|---|---|---|---|---|
| Trials | Risk of bias | Inconsistency | Indirectness of evidence | Imprecision | Other considerations | |||
| The absence of complete disease remission | CCG 1962 POG 8866 | Seriousa | Seriousb | Not serious | Not serious | Highly suspicious publication biasAll potential confounders would suggest a spurious effect, and yet no effect was observeda | LOW | CRITICAL |
| Number of postdiagnosis events | CCG 1962 DFCI 87-01 DFCI 05-001DFCI 01-91 | Seriousd | Not serious | Not serious | Not serious | Highly suspicious publication biasAll potential confounders would suggest a spurious effect, and yet no effect was observedc,d | MODERATE | CRITICAL |
| Overall mortality | DFCI 87-01DFCI 05-001 | Serious | Seriouse | Not serious | Not serious | Highly suspicious publication biasAll potential confounders would suggest a spurious effect, and yet no effect was observedc | LOW | CRITICAL |
| Hypersensitivity | CCG 1962 POG 8866 DFCI 05-001 | Serious a | Not serious | Not serious | Seriousf | Highly suspicious publication biasAll potential confounders would suggest a spurious effect, and yet no effect was observeda | LOW | CRITICAL |
CI: confidence interval; RR: relative risk.
Explanations:
The POG 8866 recruited patients previously treated with native L-Asparaginase and who were in a second bone marrow relapse and included hypersensitive patients, circumstances that may influence the response to treatment.
The protocol DFCI 87-01 used only one dose of PEG-Asparaginase; the remainder of the treatment was performed with native L-asparaginase.
The data extracted from the studies point to a similarity in the efficacy and safety between PEG-asparaginase and L-asparaginase in the treatment of ALL in children and adolescents.
In the absence of complete remission, a more significant benefit was noted for PEG-asparaginase.14,15 More individuals achieved a complete remission with PEG-asparaginase, whereas statistically fewer patients did not respond adequately to treatment (p=0.05). The statistical analysis only used the CCG 1962 and POG 886614,15 in this outcome. The POG 8866 recruited a smaller number of patients previously treated with the enzyme and who were in the second bone marrow relapse; this circumstance potentially influenced study heterogeneity and contributed to the result. According to Ettinger et al., PEG-asparaginase demonstrated efficacy similar to that of native L-asparaginase, even in the treatment of relapsed patients previously exposed to the enzyme, suggesting its use in these individuals.7
Regarding the number of postdiagnosis events calculated from event-free survival, the analysis indicated a nonsignificant result.14,16–18 Overall mortality did not exhibit a significant difference. The heterogeneity was intermediate in the analysis (I2=56%) associated with the DFCI 87-01,16 which applied only a single dose of PEG-asparaginase in the intervention group. Both asparaginase formulations are associated with high rates of event-free survival and overall survival that are comparable to each other, corroborating the statistical analysis findings.3–5
An increased tendency for hypersensitivity was noted with PEG-asparaginase, which differs from that reported in the literature.3,4,20 However, the confidence intervals in two of the three trials were high (CCG1962: 95% CI [0.25, 101.95] and POG 8866: 95% CI [0.01, 3.87]), leading to imprecision regarding these findings.14,15,17 The result was not statistically significant (p=0.68), making it difficult to state that PEG-asparaginase is associated with an increased incidence of hypersensitivity reactions.
In the analysis of antibody titers and asparaginase and asparagine serum levels, the CCG 196214 exhibited a significant difference between antibody titers between the groups in the first phase of delayed intensification (p=0.0001). In the comparison of groups, regardless of the treatment phase, native L-asparaginase participants exhibited significantly higher titers (p=0.0009).14 The POG 886615 patients who exhibited high antibody values were included in the nonrandomized patient's group. These high values are potentially related to the patient profiles, given their previous history of exposures to medication and hypersensitivity.14
Regarding asparaginase levels, the GCC 1962 (14) demonstrated that greater than 90% of the PEG-asparaginase samples exhibited levels greater than 0.03UI/mL and 0.1UI/mL. Considerably smaller estimates were noted for participants who used native L-asparaginase.14 The POG 8866 trial reported lower enzyme levels in the nonrandomized group.15 The DFCI 05-00117 reported that the intervention group obtained values greater than 0.7IU/mL throughout the period, whereas the control group reached values between 0.1IU/mL and 0.2IU/mL. PEG-asparaginase exhibits an increased half-life, compared to native L-asparaginase,21 and can be detected in the blood for up to two weeks.4,21 Thus, increased enzyme values were identified in the PEG-asparaginase samples. The POG 886615 recruited patients previously treated and included hypersensitive patients. These circumstances likely influenced antibody titers and asparaginase serum levels in these subjects. Antibody production is related to therapy efficacy, and high values may alter the therapeutic response. Antibodies can neutralize the drug, prevent its action and facilitate its clearance.3–5
The CCG 196214 demonstrated that serum asparagine decreased when asparaginase levels were greater than 0.1IU/mL,17 and this finding is related to drug activity22 and event-free survival, as reported by Moghrabi et al.23 Woo et al. demonstrated this association, revealing reductions in asparagine levels in the central nervous system with the use of asparaginase.24 The CCG 196214 also observed this reduction in the central nervous system with the two asparaginase formulations.
Although the analysis revealed the noninferiority of PEG-asparaginase, compared to native L-asparaginase, the risk of bias and quality of evidence should be assessed. Most of the trials presented a lack of information regarding the methods used, which reduces the quality of the evidence. Among the trials, only the DCFCI 05-00117 offered more research data and reduced risk of bias. The pharmaceutical industry was also involved in financing two studies (CCG 1962 and DFCI 05-001).14,17 This financing was classified in the category “other bias”.9 In the application of the GRADE system, three of the four outcomes presented a low level of evidence, suggesting the need for more detailed and careful trials.
This work aimed at research with a high level of quality, but the number of tests and their unclear methods represent limitations.
ConclusionsThis systematic review evaluated the efficacy and safety of PEG-asparaginase for ALL therapy in children and adolescents, compared to native L-asparaginase. This study concluded that PEG-asparaginase could be used as a substitute for native L-asparaginase, demonstrating a similar profile.
The assessment of the data collected was not sufficient to prove the most significant advantages in the use of PEG-asparaginase, regarding efficacy and safety, compared to native L-asparaginase. New trials with adequate methods are essential to classify PEG-asparaginase as a superior drug in the pharmacotherapy of ALL children and adolescents. However, this study opens the way for discussion and brings relevant information to decision makers of the Brazilian public health system.
Conflicts of interestThe authors declare no conflicts of interest.