Mixed phenotype acute leukemia is a rare subtype of leukemia that probably arises from a hematopoietic pluripotent stem cell. The co-expression of two of myeloid, B- or T-lymphoid antigens is the hallmark of this disease. Herein, the case of a 28-year-old female patient is reported who presented with hemoglobin of 5.8g/dL, white blood cell count of 138×109/L and platelet count of 12×109/L. The differential count of peripheral blood revealed 96% of blasts. Moreover, the patient presented with lymphadenopathy, splenomegaly and bone marrow infiltration by monocytoid blasts characterized as 7% positivity by Sudan Black cytochemical staining. Immunophenotyping revealed the involvement of blasts of both T- and monocytic lineages. The cytogenetic analysis showed an isolated 17p deletion. Thus, the diagnosis of T-cell/myeloid mixed phenotype acute leukemia was made with two particular rare features, that is, the monocytic differentiation and the 17p deletion as unique cytogenetic abnormalities. The possibility of concomitant expressions of T-cell and monocytic differentiation antigens in the same blast population is hard to explain using the classical model of hematopoiesis. However, recent studies have suggested that myeloid potential persists even when the lineage branches segregate toward B- and T-cells. The role of an isolated 17p deletion in the pathogenesis of this condition is unclear. At present, the patient is in complete remission after an allogeneic stem cell transplantation procedure.
Mixed phenotype acute leukemia (MPAL) is a heterogeneous group of rare and poorly differentiated diseases that comprises about 2–5% of all acute leukemias.1,2 This kind of leukemia can be classified as B-cell/myeloid, T-cell/myeloid, B/T-lymphoid and, more rarely, as trilineage B-cell/T-cell/myeloid.3 The T-cell/myeloid phenotype represents 35% of all MPAL.4
Most MPAL cases have been associated with cytogenetic aberrations, mainly complex karyotypes. The t(9;22) and 11q23/MLL rearrangements are the most common recurrent abnormalities. Unlike acute myeloid leukemia, with recurrent genetic abnormalities, the role of cytogenetic aberrations in the pathophysiology of MPAL remains unclear.4
Herein, the case of a patient with T-cell/myeloid acute leukemia with monocytic differentiation and an isolated 17p deletion is reported.
Case reportA 28-year-old female was admitted with a 3-week history of asthenia, fever, odynophagia and purpura. On physical examination, neck lymphadenopathy (the largest being 1.5cm), hepatomegaly (3cm below the right costal margin) and splenomegaly (10cm below the left costal margin) were found. The results of laboratory tests were as follows: hemoglobin concentration was 5.8g/dL and white blood cell count was 138×109/L. The platelet count was 12.0×109/L. A peripheral blood smear revealed 96% of blasts. Serology for B and C hepatitis, HIV, and HTLV-I/II virus were all negative.
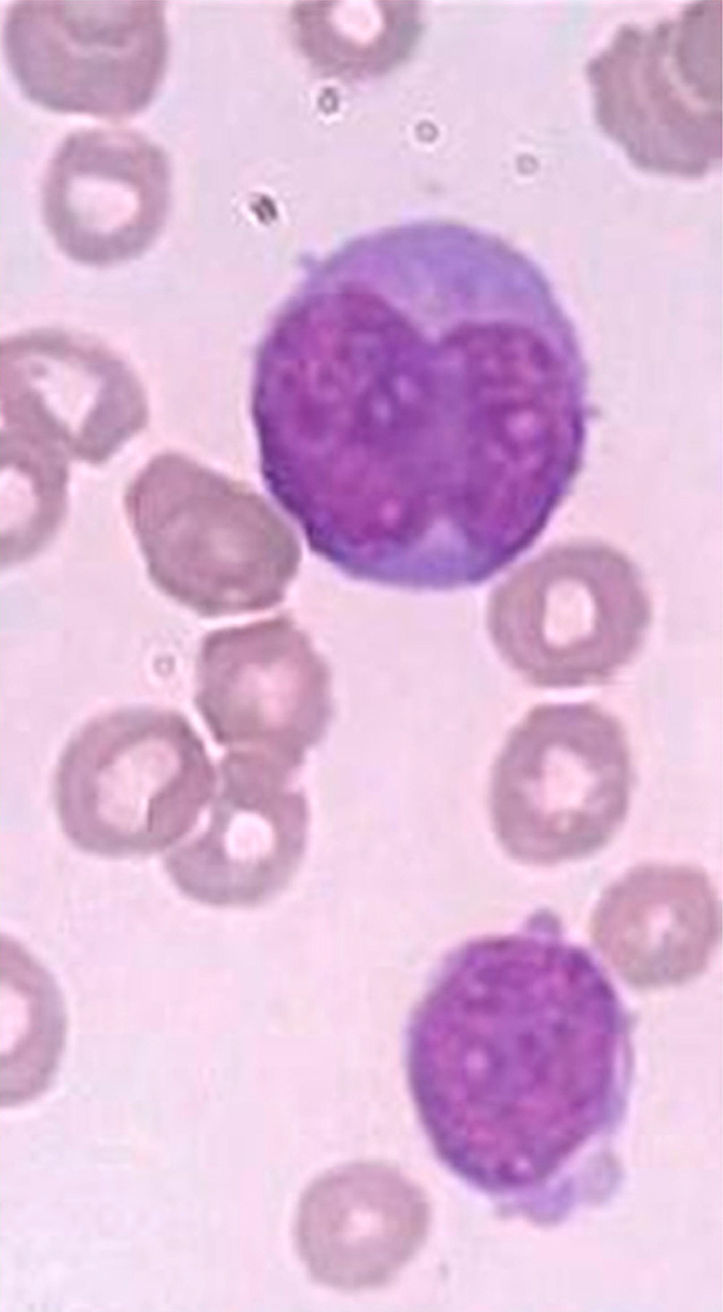
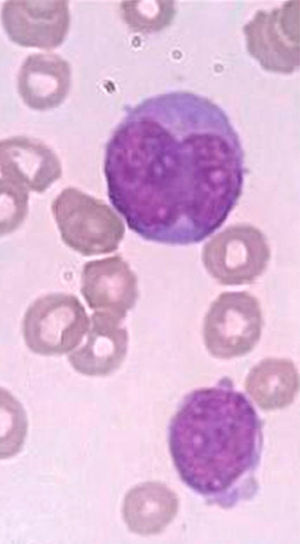
A bone marrow aspirate was performed for flow cytometry, cytogenetics and molecular biology studies. The bone marrow aspirate was hypercellular with 98% of medium- to large-sized blasts, a high nuclear-to-cytoplasmic ratio and basophilic cytoplasm. The nuclei were irregular, with a monocytoid aspect in most cells, and fine chromatin with a prominent nucleoli (Figure 1). Sudan Black cytochemical staining was positive in only 7% of blasts.
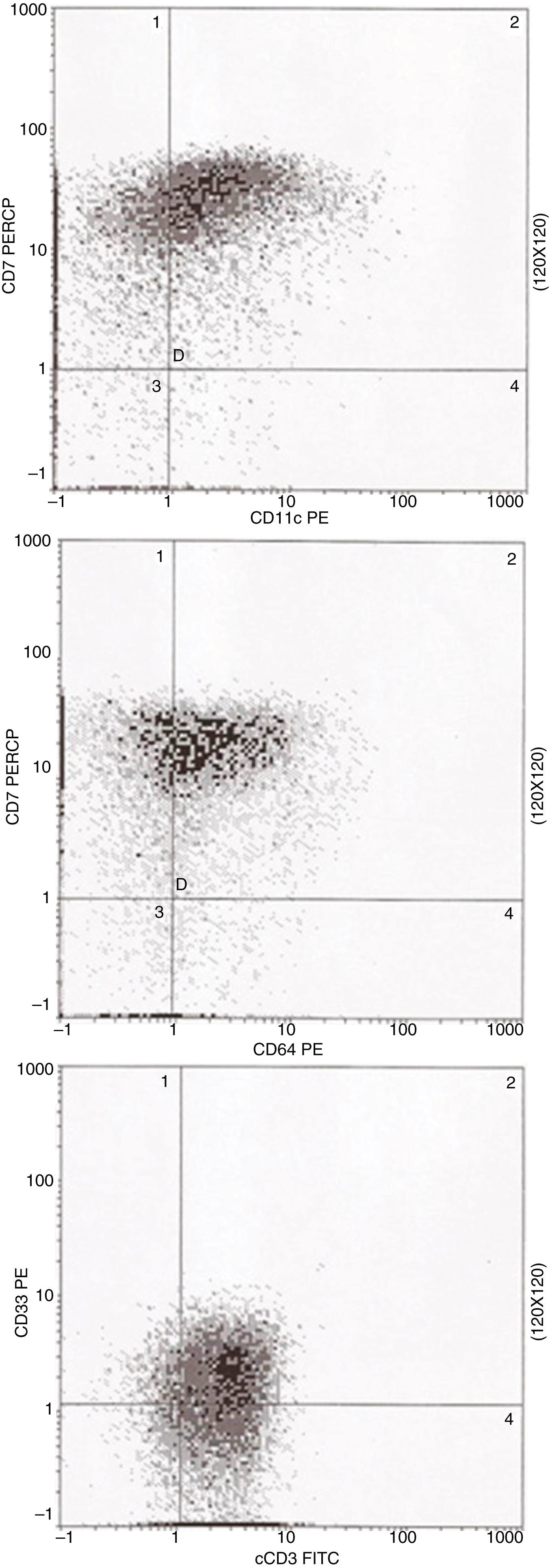
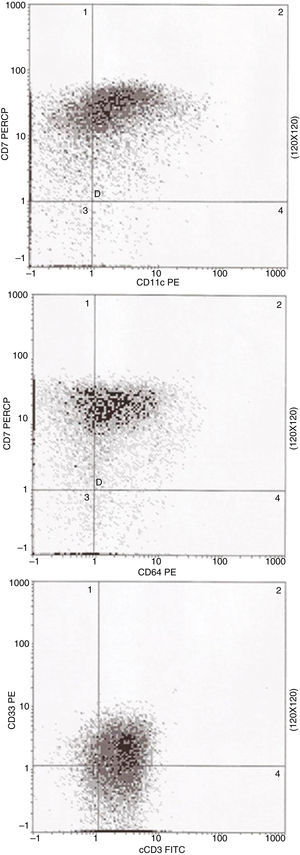
Three-color multiparameter flow cytometry analysis was performed in a Coulter EPICS XL-MCL flow cytometer equipped with an argon ion laser using the erythrocyte lyse-wash preparation method. Immunophenotyping revealed the existence of a unique population of blast cells that were positive for both monocytic and T-lineage differentiation. The blasts exhibited co-expression of monocytic differentiation markers, CD11c and CD64, together with the CD7 antigen (Figure 2). Moreover, the blasts were positive for the specific T-cell lineage intracytoplasmic CD3 (cCD3). The blasts were also positive for CD13, CD33, partial CD34, CD38, CD45weak, CD117 and partial myeloperoxidase; they were negative for CD1a, CD2, CD4, CD5, CD8, CD10, CD11b, CD14, CD19, CD56, CD61, CD79a and HLA-DR.
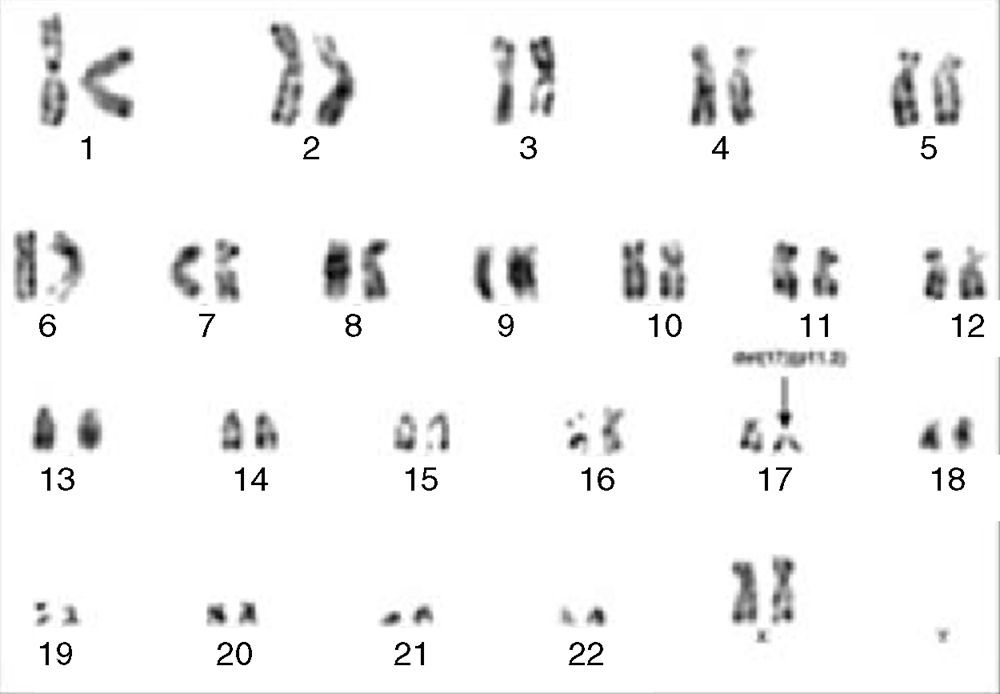
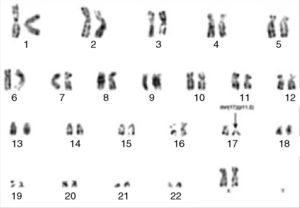
Cytogenetic analysis by G-banding showed a 46,XX,del(17)(p11.2) karyotype (Figure 3). Molecular analysis by polymerase chain reaction (PCR) of the BCR/ABL1, TEL/AML1 and MLL/AF4 fusion genes were all negative.
G-banding chromosome analysis showing isolated del(17)(p11.2). Thus, based on the 2008 revision of the WHO classification of myeloid neoplasms and acute leukemia,5 a diagnosis of MPAL, T-cell/myeloid with a monocytic component was made.
The patient was treated at the beginning with the fractionated cyclophosphamide, vincristine, Adriamycin, and dexamethasone (Hyper-CVAD) regimen and achieved hematological remission. However, at the end of the initial treatment, minimal residual disease measured by flow cytometry showed the presence of 0.03% T-cell/myeloid blasts in the bone marrow. Thus, allogeneic stem cell transplantation was performed and, at present, the patient is in complete hematological remission.
DiscussionThe 2008 WHO classification of mixed phenotype acute leukemia is based on the expression of strictly specific markers. Thus, for myeloid/monocytic lineage to be established, unequivocal myeloperoxidase positivity is necessary or at any rate, clear evidence of monocytic differentiation, which is characterized by the expression of at least two of the following markers: nonspecific esterase, CD11c, CD14, CD64 or lysozyme. The involvement of the T-cell lineage is determined by the expression of cCD3. The patient of the current study presented with blasts that only partially expressed myeloperoxidase by flow cytometry. On the other hand, the blasts clearly showed co-expression for cCD3 and the associated monocytic markers, CD11c and CD64, which immediately characterizes this case as T-cell/myeloid mixed phenotype acute leukemia with monocytic differentiation in accordance with the 2008 WHO classification.5
Mixed phenotype acute leukemia is an uncommon entity.1 The T-cell/myeloid phenotype represents about one-third of all patients with the diagnosis of MPAL.4,6 Moreover, cases of T-cell/myeloid acute leukemia with monocytic differentiation are even rarer.
Thus, in a recent published series of 100 cases of MPAL, Matutes et al. reported only four cases with CD14 expression and 13 cases showing positivity for lysozyme.4 Owaidah et al., using the criteria of the European Group for Immunophenotyping of Leukemias (EGIL), published 23 cases of biphenotypic acute leukemia (BAL) with none expressing CD14, although the CD64 antigen was expressed in three cases and the CD11b was found in seven.6 However, it is important to underscore that none of these authors mentioned whether there was concomitant expression of at least two monocytic markers in the leukemic cells as required by the WHO classification, and thus, it is actually unclear whether monocytic differentiation was really present in these series. In fact, out of 20 cases of T-cell/myeloid acute leukemia recently published in the literature, only one clearly proved monocytic differentiation.3
Interestingly, the monocytic component of the current MPAL case cannot be easily explained by the classic model of binary split differentiation between lymphoid and myelomonocytic lineages and, indeed, it is hard to justify the existence of both T-cell and monocytic involvement in the same leukemic cell. However, recent evidence has shed some light on this intricate question suggesting that in the early stages of hematopoiesis, the separation of the B-cell and T-cell lymphoid lineages may occur prior to the loss of myeloid/macrophage potential.7 Moreover, early T-cell precursors, a subset of thymocytes, are recent immigrants from the bone marrow to the thymus that retain multilineage differentiation potential, which suggests their direct derivation from hematopoietic stem cells. So, we could hypothesize that T-cell/monocytic leukemia cells arise from an immature precursor before the loss of myeloid differential potential.8,9
With regard to cytogenetic findings, chromosomal abnormalities were shown to be present in 68–91% of the cases of MPAL/BAL.4,6 Rubnitz et al. reported 35 cases of MPAL; in the T-cell/myeloid subgroup, there were just three cases with abnormalities of chromosome 17, namely, t(17,19), ins(15,17) and −17, all of them in a complex karyotype.2 Thus, as far as we know, there are no previously published cases of T-cell/myeloid MPAL with isolated 17p deletion in the literature.
In general, the 17p deletion is seen in 3–4% of acute myeloid leukemia and myelodysplastic syndromes, and confers a poor prognosis10 probably due to inactivation of the p53 gene a tumor suppression gene that plays an important role in the control of apoptosis. Approximately 32% of acute leukemia cases with del(17p) have p53 mutations. Higher percentages have been found in other hematologic malignancies.11 The role of the 17p deletion in the prognosis of MPAL remains unclear.
In summary, this is a case of T-cell/myeloid MPAL with two extremely rare features: monocytic differentiation and isolated 17p deletion.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Francisco Emiliano Rafael Dantas for the clinical patient assistance, Ronald Feitosa Pinheiro for performing cytogenetic analysis and Andrea Santos do Nascimento for technical assistance with flow cytometry.