Sickle cell anemia (SCA) is the most common monogenic multisystemic pathology in the world, affecting approximately 3.2 million people.1 It is a genetic disease with autosomal recessive inheritance, caused by a mutation in the β-globin gene, in which the nucleobase thymine is changed to adenine, leading to the replacement of glutamic acid for valine at the sixth position in the β-globin chain.2 This mutation causes structural and functional modifications in erythrocytes, which have their membrane distorted under deoxygenated conditions, leading to vaso-occlusion and extravascular hemolysis.3
Vaso-occlusion and hemolysis cause numerous pathological events, which can lead to a wide range of complications. Among the neurological ones, stroke is the most common, affecting around 11 % of SCA patients before the age of 20 years old.4 Spontaneous epidural hematoma is an extremely rare complication of the disease.5
We present a case of a patient with sickle cell anemia and spontaneous epidural hematoma (EDH), emphasizing the difficulty of diagnosis due to the rarity of the event and the benefit of early recognition in the patient's evolution.
Case reportAn 11-year-old boy with sickle cell anemia (HbSS) was admitted to the emergency department of Hospital Infantil Darcy Vargas in São Paulo with a history of tonic-clonic seizure. After the seizure, the patient remained drowsy and with dysarthria. A day before the admission, the patient had presented intense leg pain and was treated with painkillers at home. There was no history of head trauma.
On physical examination, the patient was tachycardic, pale, eupneic, afebrile, Glasgow Coma Scale score of 15/15, isocoric and photoreactive pupils, no palpable skull fractures, no focal deficits.
Laboratory tests showed hemoglobin (Hb) of 7.8 g/dL; leukocytes 22,700/mm3, without left shift; platelets 369,000/mm3; reticulocytes 13 % and with normal coagulogram. A CT scan of the skull showed a small hyperdense extra-axial collection located in the right parietal high convexity with no evidence of bone fractures or midline shift.
Twelve hours after the hospital admission, the patient presented fever, decrease of consciousness level (Glasgow Coma Scale score of 12/15), periorbital edema and bilateral proptosis of the eyeballs, along with a large subgaleal hematoma that was not present at the admission. New laboratory tests showed worsening anemia with Hb of 4.7 g/dL and decreased platelets, 126,000/mm3. The patient received a transfusion of red blood cells, with a post-transfusion Hb of 8.0 g/dL.
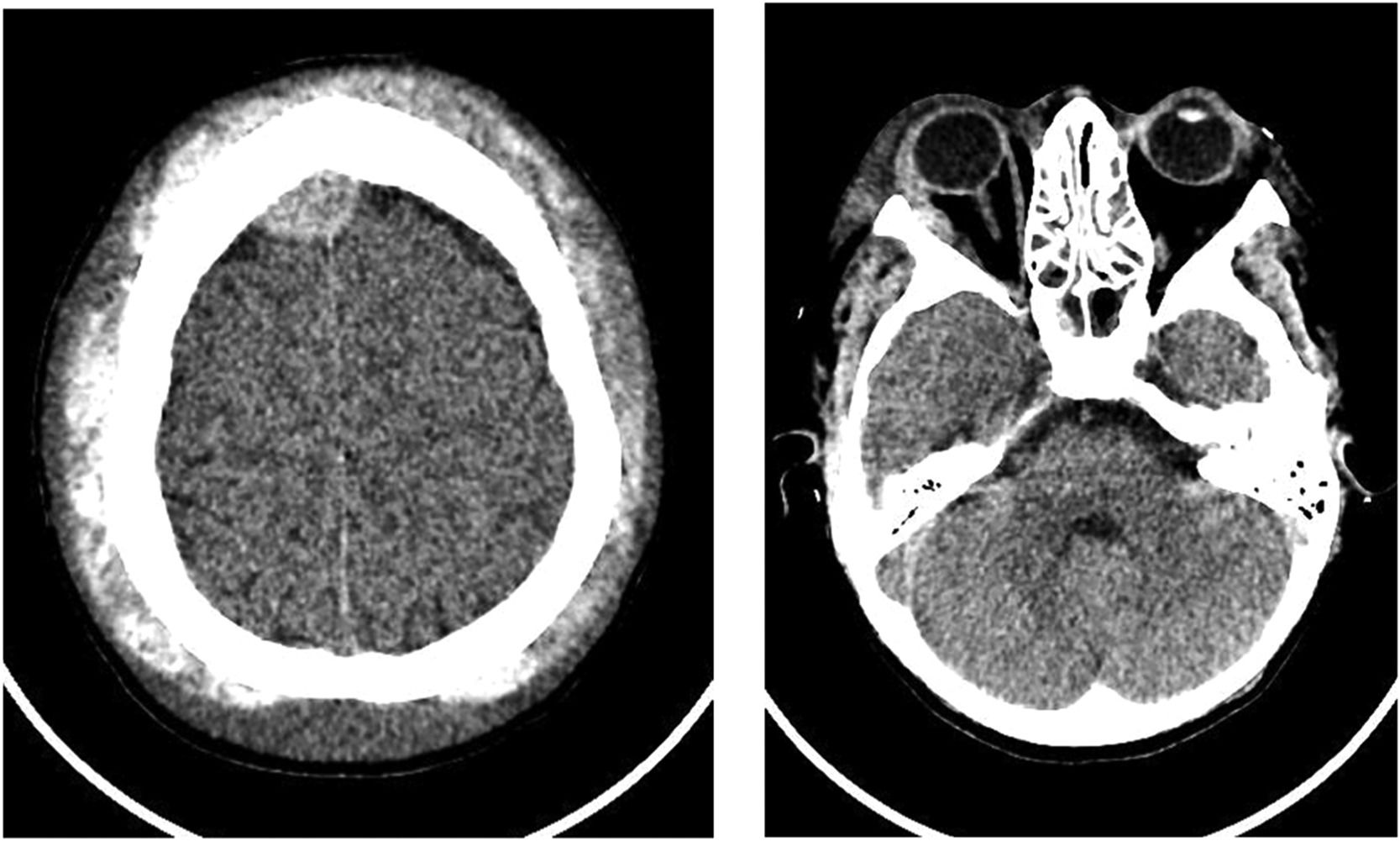
A new CT scan of the skull showed a epidural hematoma in the right frontoparietal high convexity, with 10 mm thickness, pressing the adjacent parenchyma, associated with dense subperiosteal collections near both orbits, two on the right and one on the left, with thicknesses of 11 mm and 10 mm respectively, promoting proptosis of the eyeballs (Figure 1).
Neurosurgical evaluation was requested and magnetic resonance imaging of the skull confirmed the frontoparietal epidural hematomas and extraconal intraorbital hematomas, as well as signs of bone infarction and diffuse heterogeneity of skull's bone marrow. A hypothesis of non-traumatic spontaneous epidural hematoma related to sickle cell anemia was made.
Expectant management was chosen by the neurosurgery team. The patient gradually improved from the epidural and intraorbital hematomas, with partial regression of the ocular proptosis. He remained hospitalized for 15 days, receiving antibiotic therapy due to the initial fever. Two weeks later, the patient returned for an outpatient visit, with complete regression of the hematomas and ocular proptosis.
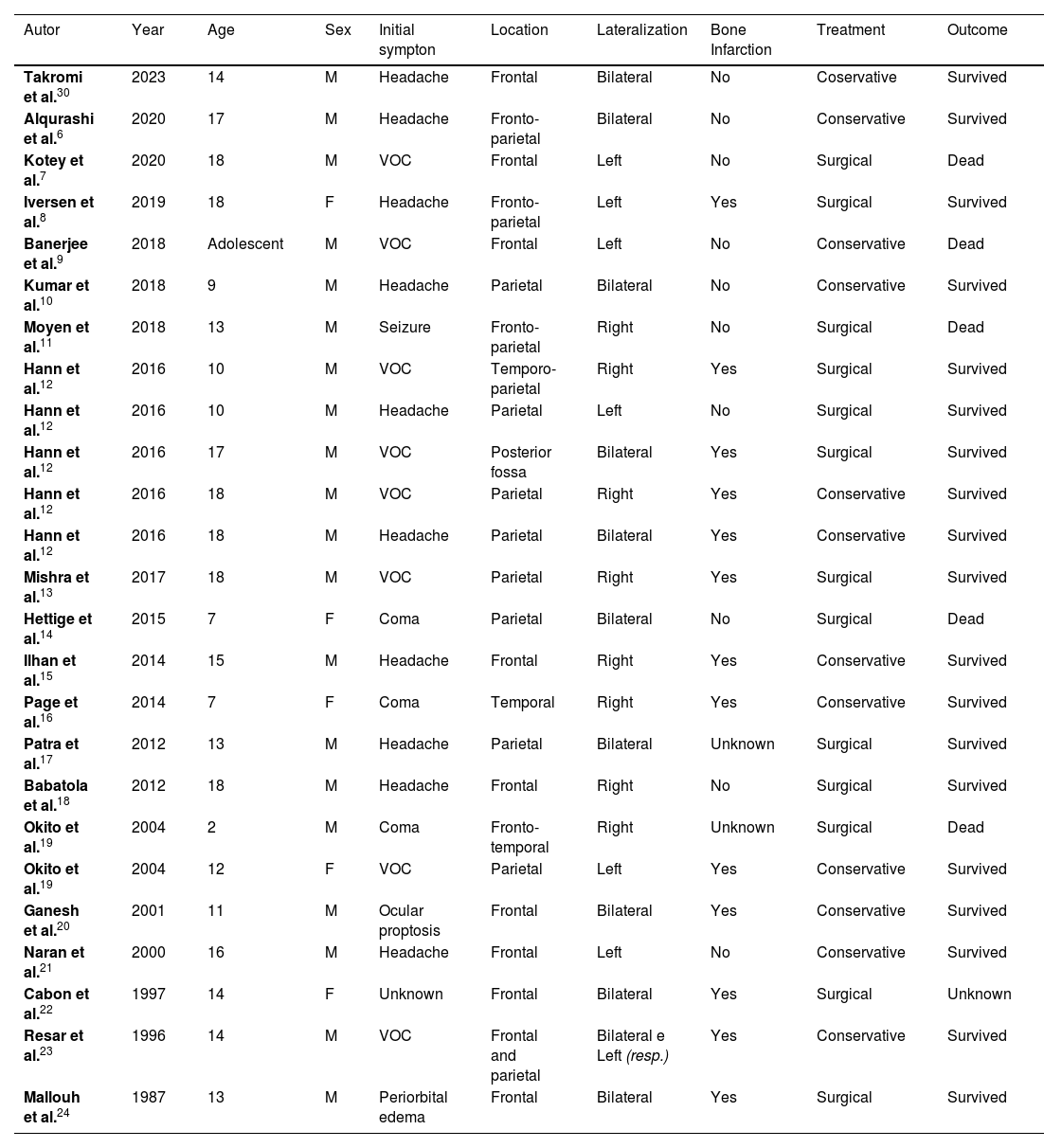
DiscussionWe describe a rare case of EDH in a pre-adolescent with SCA and we found 25 cases reported in the literature in pediatric patients up to 18 years old, with SCA (HbSS), as shown in Table 1.
Pediatric cases reported of EDH in association with SCA.
| Autor | Year | Age | Sex | Initial sympton | Location | Lateralization | Bone Infarction | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Takromi et al.30 | 2023 | 14 | M | Headache | Frontal | Bilateral | No | Coservative | Survived |
| Alqurashi et al.6 | 2020 | 17 | M | Headache | Fronto-parietal | Bilateral | No | Conservative | Survived |
| Kotey et al.7 | 2020 | 18 | M | VOC | Frontal | Left | No | Surgical | Dead |
| Iversen et al.8 | 2019 | 18 | F | Headache | Fronto-parietal | Left | Yes | Surgical | Survived |
| Banerjee et al.9 | 2018 | Adolescent | M | VOC | Frontal | Left | No | Conservative | Dead |
| Kumar et al.10 | 2018 | 9 | M | Headache | Parietal | Bilateral | No | Conservative | Survived |
| Moyen et al.11 | 2018 | 13 | M | Seizure | Fronto-parietal | Right | No | Surgical | Dead |
| Hann et al.12 | 2016 | 10 | M | VOC | Temporo-parietal | Right | Yes | Surgical | Survived |
| Hann et al.12 | 2016 | 10 | M | Headache | Parietal | Left | No | Surgical | Survived |
| Hann et al.12 | 2016 | 17 | M | VOC | Posterior fossa | Bilateral | Yes | Surgical | Survived |
| Hann et al.12 | 2016 | 18 | M | VOC | Parietal | Right | Yes | Conservative | Survived |
| Hann et al.12 | 2016 | 18 | M | Headache | Parietal | Bilateral | Yes | Conservative | Survived |
| Mishra et al.13 | 2017 | 18 | M | VOC | Parietal | Right | Yes | Surgical | Survived |
| Hettige et al.14 | 2015 | 7 | F | Coma | Parietal | Bilateral | No | Surgical | Dead |
| Ilhan et al.15 | 2014 | 15 | M | Headache | Frontal | Right | Yes | Conservative | Survived |
| Page et al.16 | 2014 | 7 | F | Coma | Temporal | Right | Yes | Conservative | Survived |
| Patra et al.17 | 2012 | 13 | M | Headache | Parietal | Bilateral | Unknown | Surgical | Survived |
| Babatola et al.18 | 2012 | 18 | M | Headache | Frontal | Right | No | Surgical | Survived |
| Okito et al.19 | 2004 | 2 | M | Coma | Fronto-temporal | Right | Unknown | Surgical | Dead |
| Okito et al.19 | 2004 | 12 | F | VOC | Parietal | Left | Yes | Conservative | Survived |
| Ganesh et al.20 | 2001 | 11 | M | Ocular proptosis | Frontal | Bilateral | Yes | Conservative | Survived |
| Naran et al.21 | 2000 | 16 | M | Headache | Frontal | Left | No | Conservative | Survived |
| Cabon et al.22 | 1997 | 14 | F | Unknown | Frontal | Bilateral | Yes | Surgical | Unknown |
| Resar et al.23 | 1996 | 14 | M | VOC | Frontal and parietal | Bilateral e Left (resp.) | Yes | Conservative | Survived |
| Mallouh et al.24 | 1987 | 13 | M | Periorbital edema | Frontal | Bilateral | Yes | Surgical | Survived |
VOC: vaso-occlusive crisis.
Since the first report in 1987, the incidence of spontaneous epidural hematoma in children and adolescents has increased dramatically. This does not necessarily reflect a real increase in the number of cases, but rather a radiology advancement and an increased awareness about the subject, since there is greater access to academic content and more opportunities for publication. Nonetheless, even after more than 30 years, there are few reports of spontaneous epidural hematoma in pediatric patients with sickle cell anemia (HbSS).5
Most of the reports are from pre-adolescent and adolescent male patients. The most prevalent symptoms at the admission were headache in 39 %, followed by vaso-occlusive crisis in 34 %, and decreased level of consciousness in 13 %. Other reported symptoms included convulsive crisis, periorbital edema, and ocular proptosis. Despite our patient having started with a convulsive crisis, the presence of vaso-occlusive crisis in the lower limbs was reported the day before admission, progressing to periorbital edema and ocular proptosis 12 h after hospitalization, with only two other cases in the literature by Mallouh et al.24 and Ganesh et al.20 reporting this presentation.
The most common locations of the EDH were the frontal and parietal regions, each accounting for 36 %. The predominant laterality was right in 32 % of the hematomas, and bilateral involvement was present in 40 % of the cases. The reported patient presented with a hematoma in the right frontoparietal region, with only one other report in the literature by Moyen et al.11 of a patient with EDH in the same location and laterality.
The pathophysiology of epidural hematoma in patients with sickle cell anemia is not well understood. Around 60 % of the reports identified bony infarcts of the skull in the same area as the hematoma. Therefore, it is speculated that there may be underlying bone vasospasm with periosteal elevation and vessel wall rupture, leading to bleeding into the epidural space.
Dahdaleh et al. elucidated an alternative pathophysiological mechanism, suggesting that chronic extramedullary hematopoiesis, observed in sickle cell disease, contributes to cortical thinning of the skull bones and expansion of the bone marrow. In response to acute anemia, rapid proliferation and expansion of hematopoietic tissue would result in the rupture of cortical vessels, precipitating the extravasation of blood into the epidural space. Finally, insufficient venous drainage would lead to congestion and excessive edema, causing epidural hemorrhage.25
We believe that the two mechanisms could have contributed to the EDH in the reported patient, as adjacent bone infarctions were observed alongside the hematoma, and there was a sudden drop in hemoglobin levels. This drop could stimulate extramedullary hematopoiesis, supported by the diffuse heterogeneous appearance of the cranial bone marrow as seen in the cranial MRI.
Another important factor for the etiology of EDH in patients with SCA would be the presence of thrombocytopenia and other coagulopathies. However, the patient's laboratory tests did not reveal such abnormalities.26 Involvement of the ocular globes is extremely rare in these cases. It is postulated that blood accumulates in the superior orbital ridges, which could disrupt the attachment of the arcus marginalis muscle, leading to the buildup of blood within the orbital cavity. This can result in exophthalmos, decreased vision, and ophthalmoplegia.27
Interestingly, the literature shows that patients which were identified bone infarctions had a higher survival rate than those who did not. This event may indicate that vaso-occlusive etiology of the EDH has a better prognosis. Identifying bone infarction can be challenging, and MRI seems to be the most sensitive tool, while CT has low efficacy, especially in the acute phase of the disease.9
Management and treatment of EDH depend on the volume, level of consciousness, and hemodynamic status. In the last decades more and more studies reported that the conservative treatment has been chosen for small epidural hematomas. However, this approach requires a close neurological observation and serial CT scanning.28
Generally, epidural hematomas less than 30cm3, less than 15 mm in thickness, less than 5 mm midline shift, a Glasgow Coma Scale score greater than 8 and without focal deficit could be managed conservatively. In case of any of these parameters change, the surgical treatment should be considered, due to the risk of herniation.29
Among the cases reported in the literature, 45 % were managed conservatively, and 55 % required surgical intervention. It was observed that patients who underwent conservative treatment had a survival rate of 91 %, while those who underwent surgical treatment had a survival rate of 61.5 %. The overall mortality among the cases was 22 %.
ConclusionThis case illustrates a rare neurological complication and emergency of sickle cell anemia in a pediatric patient. We believe that it will elucidate the clinical and pathophysiological aspects of the disease, since it is an important differential diagnosis of other neurological and vaso-occlusive conditions. This case will help in an early recognition of patients with this pathology reducing the risk of deterioration and formulating a care plan based on the patient's evolution.