The anti-Jra antibody acts against a high-frequency (HF) antigen and is rarely seen clinically. It exhibits positive reactions on all antibody screening and identification cells but negative reactions to auto cells and also negative results in the direct antiglobulin test (DAT). Anti-Jra IgG can transfer through the placenta and cause transiently positive unexcepted antibody in neonates.1-4 As Jra is a HFA, most populations are Jra-positive. However, whether neonates will positive in DAT and elution test, whether anti-Jra positivity results in hemolytic disease of the fetus and newborn (HDFN), and the serological changes in neonatal blood group substances caused by anti-Jra and their clinical significance have seldom been reported in Chinese patients. Here, we report a case in which anti-HFA antibodies were detected during pregnancy and the anti-Jra antibodies caused transiently positive free antibody test results in the neonate. Major pathophysiological changes leading to hemolytic disease of the fetus and newborn (HDFN) caused by antibodies to the HFA were searched in databases such as PubMed and CNKI.
Summary of the case courseNeonateThe mother was gravida 4, para 1. One child had died of congenital heart disease in 2014, two medical abortion had been performed at week 7–8 of pregnancy in 2015 and 2018. At 28 weeks +6 of the current pregnancy, the mother was admitted to the hospital with fetal pericardial effusion and tricuspid regurgitation, and anti-HFA unexcepted antibodies were detected. Specific antibody identification was not performed, and the fetus was not monitored or treated. The neonate was then delivered by cesarean section at 37 weeks +2 under general anesthesia, and the postnatal Apgar score was 7 at 1 min of life (respiration:–1, laryngeal reflex:–1, skin color:–1) and 9 at 5 min (respiration:–1). The birth weight was 3360 g. Physical examination at admission revealed:temperature, 36.3 °C; pulse, 130 beats/min; respiration, 46 breaths/min; weight, 3360 g; blood pressure, 69/40 mm Hg; and transcutaneous oxygen saturation without oxygenation, 90%. The neonate was conscious after birth, slightly unresponsive, and had a slightly pale body color. There were coarse breath sounds on auscultation of both lungs, with no dry or wet rales. Heart beats were strong and rhythmic, with no murmurs. The abdomen was soft and not inflated, the muscle tone in the limbs was normal, and there was no hepatosplenomegaly or edema. Blood tests revealed:postnatal hemoglobin concentration, 136 g/L; reticulocytes, 6.61%; total bilirubin level, 57.5 µmol/L; and indirect bilirubin level, 45.1 µmol/L. Cardiac ultrasound indicated patent ductus arteriosus, predominantly left-to-right bidirectional low-velocity shunting at the level of the aorta, tricuspid regurgitation, and a small left-to-right shunt at the level of the atria. After admission, the neonate was provided oxygen using a humidified high-flow nasal cannula (flow rate, 4 L/min; FiO2, 0.21). After 1 h, breathing was stabilized and blood gas values normalized. After 1 day, the neonate was weaned from the respirator and monitored. Transcutaneous oxygen remained above 93%. Transcutaneous bilirubin and serum total bilirubin levels were normal. On day 7 after birth, there was no respiratory distress, apnea, drowsiness, cyanosis, or peripheral edema and transcutaneous bilirubin was 80 µmol/L. The neonate was not treated with phototherapy or blood transfusion during hospitalization and was discharged.
Maternal and neonatal blood typing serologyMaternal and neonatal ABO typingThe mother and neonate were both type B, RhD-positive.
Neonatal and maternal unexcepted antibody screeningNeonatal and maternal red bloods cells were screened for unexcepted antibodies using microcolumn gels (Baso Biotechnology, Zhuhai, China) and unexcepted antibody screening cells (Blood Biomedical, Shanghai, China). All results were positive.
Identification of neonatal and maternal unexcepted antibodiesNeonatal and maternal antibodies were identified using microcolumn gels (Baso Biotechnology, Wuhan , China), and antibody were identified using 16 cells panel (Sanquin, Amsterdam, The Netherlands). All results were positive, with agglutination intensities of 1+ to 2+. Maternal DAT was negative, suggesting antibodies to HFAs.
Antibody titer determinationAntibody titer in maternal and neonatal sera were determined using microcolumn gels (Baso Biotechnology). Cells with an agglutination intensity of 2+ in antibody identification were used. The antibody titer was 32 for both the mother and neonate.
Enzyme and dithiothreitol (DTT) treatmentMaternal serum was treated with papain (Bio-Rad, USA) and 0.2 M DTT (prepared in-house) respective before antibody identification. The identification results were positive, indicating the antibody is papain and DTT resistance.5
HDFN examinationThe serum of the neonate tested positive for free antibodies, very weakly positive for DAT, and warmer elution PeG-IAT result shows negative, acid elution PeG-IAT result shows positive
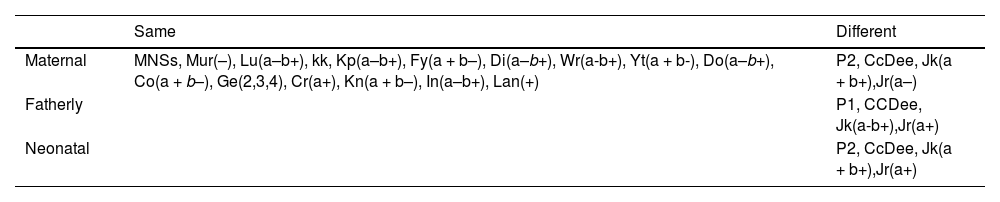
Parents blood group antigen testingUsing BLOODchip v4.1 (Progenika, Vizcaya, Spain)6,7 detected parents and the neonatal blood group gene. Result shows maternal lack the HFA Jr(a) gene, and fatherly and neonatal contain the Jr(a) gene (Table 1).
Confirmation of anti-Jra antibodiesMaternal serum, neonatal serum and acid-elution exhibited a negative reaction with known Jra-negative frozen red blood cells.
DiscussionIn this paper, we reports a neonate and his mother with anti-Jra in serum, showed 2+ agglutination with all panel cells, titer were 32 as the characterized as high-titer, low-affinity antibodies(HTLA.But very weak DAT results and negative in warmer elution, only positive in acid elution. And the neonate showed no anemia.
A review of the literature indicates that anti-Jra may be clinically significant because it has been implicated various in cases of HDFN and transfusion-related hemolysis.5,7-13
The Jr blood group system currently contains only one antigen, Jra, which is expressed on the ABCG2 glycoprotein, a multi-pass transmembrane protein of 655 amino acids. ABCG2 is an ATP-dependent transporter protein with high substrate diversity and is highly expressed on erythrocytes and the chorion.5,8 Jra is a HFA, but its density varies among individual erythrocytes.8,9 Jra negativity (Jra(–)) is mostly due to frameshift or nonsense mutation. Common mutations include c.1515delC, c.376C>T, c.421C>A, and c.1723C>T.7
Anti-Jra antibodies were first reported in 1970 by Stroup, who reported seven cases, including three Japanese1. Subsequently, Jra(–) was found to be more prevalent in the Japanese population, with a prevalence of 0.03–0.12%, which is higher than in the USA, Mexico, and the Middle East, but seldom report in Chinese.1,10-12 The majority of anti-Jra antibodies are produced during pregnancy; only a minority are produced after blood transfusion. Most anti-Jra antibodies is IgG, mainly of the IgG1 and IgG3 subclasses, and anti-Jra antibodies can bind complement. There are only two reports of naturally occurring anti-Jra IgM.1,10 In Japan, anti-Jra antibody is the fourth most prevalent alloantibody in pregnant women after anti-E, anti-M, and anti-D, and the proportion of anti-Jra is higher in women than in men.11,12
There are few reports of acute hemolytic reactions due to incompatible anti-Jra transfusion. Kwon et al.13 reported two patients with anti-Jra due to blood transfusion. One woman with a history of pregnancy developed fever and had increased lactate dehydrogenase levels and hemoglobinuria after the transfusion of three units of minimally incompatible Jra(+) red blood cells, establishing the presence of an acute hemolytic transfusion reaction. Another patient who received the same minimally incompatible transfusion regimen for gastrointestinal bleeding did not demonstrate hemolysis, but developed Fyb antibodies after the transfusion and had a delayed serological reaction. In both patients, the antibody titers increased after transfusion (from 32 to 6400 and from 32 to 128, respectively). The two patients presented with different symptoms, which may be because of the different densities of Jra-positive red blood cell antigens.
There are few reports of HDFN caused by anti-Jra. Most cases of HDFN caused by anti-Jra are mild and positive in DAT and/or eluation tests, with or without neonatal jaundice and most of them do not require blood transfusion. However, mothers with highly titer anti-Jra may experience preterm delivery, intrauterine fetal death, or severe fetal anemia.14 In Japan, severe fetal anemia has been reported in approximately 25% of pregnant women with anti-Jra.15,16 There are three reasons why anti-Jra rarely causes HDFN in the early reports. First, although both IgG1 and IgG3 are believed to be able to transfer through the placenta, IgG1 may be less likely to cause HDFN. Second, the density of Jra antigen on the surface of fetal erythrocytes is low. Third, the anti-Jra antigen-antibody response may be dose-dependent. HDFN develops when anti-Jra are of the IgG3 subtype and the red blood cell antigen density is high; otherwise, severe HDFN does not develop.14
Kendall et al.17 showed that when 51Cr-labeled Jra(+) red blood cells were transfused into volunteers with anti-Jra, the Jra(+) red blood cells were rarely detectable after 24 h, indicating a shortened red blood cell survival period after antibody sensitization.18 Studies have focused on the anti-Jra titer, IgG subtype, monocyte monolayer assay (MMA)values, and fetal peak middle cerebral artery peak systolic velocity (MCA-PSV) to predict the occurrence of HDFN.19-25 Ishihara et al.19 reported a case of anti-Jra-induced HDFN that resulted in severe anemia. The 29-week obstetric examination of a pregnant woman with anti-Jra revealed elevated fetal MCA-PSV, fetal edema, and fetal hemoglobin concentration of 23 g/L. After three intrauterine transfusions at 30, 33, and 34 weeks, the neonate was delivered at 35 weeks with a hemoglobin concentration of 72 g/L. After delivery, the newborn required transfusion, but did not develop hyperbilirubinemia. Arriaga et al.20 reported the case of a Spanish woman (gravida 5, para 3) in whom anti-Jra antibodies were detected before the second pregnancy, MMA was normal, and IgG was of the IgG4. The antibody titer was 8. The third and fourth pregnancies resulted in DAT-positive neonates in whom anti-Jra were detected; however, they did not develop HDFN. In the fifth pregnancy, the antibody titer was 128, IgG was of the IgG2 and IgG3 types, and the MMA test result was 15% (control:7%), this fetus was aborted. This case suggests that a history of multigravidity, elevated anti-Jra titer during pregnancy, IgG3 subtype, and positive MMA suggest an increased risk of HDFN, and MCA-PSV can be used to predict the degree of fetal anemia.
Kanagawa et al.21 reported the case of a pregnant woman with anti-Jra and investigated whether the anti-Jra titer affects MCA-PSV and can thus predict fetal anemia. It is generally believed that the risk of fetal anemia increases when the anti-D titer is 16. In fetuses with severe anemia, once intrauterine transfusion has been administered, elevated MCA-PSV may suggest severe anemia, and the fetus may require repeated intrauterine transfusions. Therefore, the authors adopted the same monitoring protocol as for anti-D antibodies, i.e., antibody titer measurements every 4–6 weeks and MCA-PSV every 2 weeks. When the multiple of the median (MoM) exceeds 1.5, indicating the fetus has anemia. Intrauterine transfusion with type O and Jra-matched red blood cells was performed. MCA-PSV greater than 1.5 MoM at re-examination before 34 weeks suggests that intrauterine transfusion has to be repeated, and if severe anemia occurs after 34 weeks, the pregnancy should be terminated.
To determine the threshold value for anti-Jra-induced fetal anemia, pregnant women with anti-Jra between 2010 and 2017 were retrospectively investigated, and the maternal antibody titer, maximum antibody titer, and MCA-PSV at different weeks of gestation, cord blood hemoglobin, intrauterine transfusion, and mode of delivery were analyzed.21 Of the 16 women investigated, six women received intrauterine transfusion at 27–32 weeks and 2 experienced miscarriages. In one case, MCA-PSV exceeded 1.6 MoM at 34 weeks, the antibody titer increased from 32 to 64, and umbilical cord blood puncture revealed a Hb level of 90 g/L. No cases of MCA-PSV exceeding 1.5 MoM after 32 weeks were observed. 15 pregnancies were delivered at term and one at 36 weeks. No neonates developed anemia or jaundice, and no transfusion or phototherapy was performed. The antibody titers in neonates who did and did not receive intrauterine transfusion were 8–4096 and 2–2048 respectively, with no statistically significant difference. This retrospective study did not give a certain anti-Jra titer threshold for intrauterine transfusion. Ohto et al.22 found that the severity of anemia, jaundice, and the need for treatment of HDFN caused by anti-Jra were not related to the antibody titer, unlike HDFN caused by anti-D. Most children did not exhibit elevated reticulocyte counts or compensatory hematopoiesis enhancement.
Kanagawa et al.21 and Toshimitsu et al.23 found that most cases of severe anemia occur before 32 weeks. When maternal antibody titers increase, IgG1 and IgG3 antibodies may lead to severe fetal anemia, with Hb levels of or below 50 g/L, indicating the requirement for intrauterine blood transfusion. However, even if intrauterine transfusion was administered before 32 weeks, MCA-PSV suggested that anemia did not aggravate after 32 weeks and full-term delivery was possible without severe neonatal anemia. If anemia is detected after 37 weeks, early delivery may not be needed. Anti-Jra antibodies do not exacerbate fetal anemia in the third trimester, suggesting that the mechanism of HDFN caused by anti-Jra differs from that of HDFN caused by other blood group antibodies. Our patient underwent screening for unexcepted antibodies against red blood cells at a gestational age of 28 weeks +6, and we detected antibodies to HFAs. As antibody specificity was not determined at the time, no clinical intervention was conducted. The neonate was delivered through cesarean section at 37 weeks and showed mild anemia after birth without hyperbilirubinemia. The neonate did not receive transfusion or phototherapy and was discharged after remission. However, it is not clear whether prenatal ultrasonography suggested the fetal cardiac dysplasia related to anti-Jra.
Ohto et al.22 retrospectively investigated the different mechanisms of HDFN caused by anti-Kell, anti-M, anti-Ge3, and anti-Jra. As Jra antigens were highly expressed on placental villi, they suggested that Jra may be an antigen during early placental development and anti-Jra may enter fetal circulation via the placenta, leading to a decrease in sensitized erythrocyte and less severe fetal anemia. To investigate the mechanism of anti-Jra-induced fetal anemia further, Kanagawa et al.21 investigated differences in the expression levels of ABCG2 in fetal erythrocytes at different gestational ages. ABCG2 is a cell membrane transporter and epitope of the Jra antigen and is thought to be responsible for maintaining the undifferentiated state in hematopoietic stem cells. It was found that before 32 weeks of gestation, high ABCG2 expression levels in erythroid progenitor cells may lead to a high affinity for anti-Jra, resulting in severe fetal anemia. However, after 32 weeks, erythropoietic progenitors gradually differentiated into mature erythrocytes with low ABCG2 expression and anemia did not continue to worsen, unlike the gradual increase in D antigen expression during late pregnancy. Thus, patients who received intrauterine transfusion before 32 weeks can deliver at term after 32 weeks.24,25
Another reason that the antibody titer is not related to anemia may be that ABCG2 expression varies among individuals. Although ABCG2 expression is 1–4 times higher in fetuses than in adults, there are large individual differences, and the amount of ABCG2 in umbilical cord blood erythrocytes varies among individuals of the same gestational age neonates.21 Endo et al.14 reported a case of transient dyspnea and leukopenia in a neonate whose mother carried anti-Jra. The mother did not develop anemia or hyperbilirubinemia during the first pregnancy and the results of biochemical tests were normal; therefore, no treatment was administered. The second fetus presented with dyspnea and decreased oxygen saturation, and neonatal red blood cells exhibited weak positivity to maternal serum after adding polyethylene glycol. The PCR-SSP method was used to detect the presence of base substitutions at positions 376 and 421 in ABCG2, which are believed to be the main factors affecting Jra antigen density, in both the parents and their two children. The results revealed base substitutions in both children, with the first child being a 376 C/T heterozygote with a C>A mutation at position 421 and the second child being heterozygous only for 376 C/T. Flow cytometric detection of Jra antigen density in the family members revealed 31% expression of normal in the first child and 69% expression in the second child as compared to red blood cells with normal Jra expression. Jra antigen expression differed between the two children. Analysis of Jra antigen density in the two children revealed that the first child had low antigen density and was asymptomatic, whereas the second child had high antigen density and had symptoms of respiratory distress. Bone biopsies of the second child on the 4th and 5th days after birth revealed bone marrow hyper-differentiation on day 5, suggesting that maternal anti-Jra may affect neonatal Jra antigen density and that the red blood cells produced by hyper-differentiated bone marrow have low Jra antigen expression. As the maternal IgG antibodies lasted in the neonate for 3 months, the neonate phenotype was Jra(+) on re-examination after 3 months, which was inconsistent with the weak positivity at birth. The haptoglobin level also returned to normal at 3 months after a decrease in the postnatal period. Bone biopsy at 4 months indicated disappearance of bone marrow hyper-differentiation. Ohto et al.22 described a case of a neonate who was Jra(–) at 3 days of life, with weakly positive elution fluid. By 1 month, the Jra antigen showed as mixed appearance and at 10 months, the child tested positive for Jra, suggesting that high-titer anti-Jra may cause clonal escape through epistatic modification of RNA or DNA. This situation is common in high-titer anti-Jra and may be a reason why high-titer anti-Jra produce milder symptoms.21 ABCG2 is expressed not only on erythroid lineage cells, but also in the placenta, where it is an endogenous and exogenous substrate of plasma membrane proteins. ABCG2 maintains a stable internal environment of cytosolic porphyrins and heme (the basic components of hemoglobin) and plays an important physiological function in erythropoiesis. Anti-Jra may directly affect the function of ABCG2, producing ineffective red blood cells, leading to fetal anemia.24,25
In summary, the mechanism of fetal anemia caused by anti-Jra antibodies differs from that of fetal anemia caused by other antibodies and is related to the level of Jra antigen expression on fetal red blood cells. Anti-Jra may inhibit Jra antigen expression on the surface of red blood cells, resulting in transiently negative Jra antigen results in the neonate. Fetal anemia caused by anti-Jra occurs mostly in the second trimester of pregnancy when Jra is highly expressed and does not worsen after 32 weeks. If intrauterine transfusion is administered before 32 weeks, the fetus can be delivered at term, the newborn may be DAT-negative or -positive, and most infants do not develop hyperbilirubinemia and do not require treatment with blood transfusion(s). Anti-Jra may cause anemia independent of the antibody titer, and fetal anemia is predicted by the IgG subclass, MMA activity, number of pregnancies, and increased MCA-PSV.
This study was supported by the Liaoning Science and Technology Program (grant numbers 2018225088 and 2021JH2/10300045).
We would like to thank Editage (www.editage.cn) for English language editing.