The RHD gene is responsible for the expression of the D antigen while RHCE encodes proteins carrying antigens C or c, and E or e. RHD is present or absent depending on the RH haplotype, and RHCE displays four common alleles (ce, Ce, cE, CE) responsible for the expression of the two antithetical (allelic) series of antigens. RHD gene deletion is the leading cause of the D-phenotype worldwide.1 Functional (antigen) variations in the Rh blood group system is determined by insertion/deletion, single nucleotide polymorphisms (SNPs), and gene conversion events in the RHD and RHCE genes. A weak D type is a variant of the RhD protein that comprises an amino acid substitution located in the transmembraneous or intracellular segments and expresses a reduced amount of the D antigen [generally less than 5000 D antigens per red blood cell (RBC)].2,3
The RHD*weak D type 38 is produced by the single nucleotide change (833G>A) in the exon 6, causing a Gly278Asp substitution in the transmembraneous RhD protein. This RHD allele shows reduced expression of the D antigen on the RBC surface and may be erroneously typed as D negative by standard serologic methods.4–6 The frequencies of weak D type 38 vary in different populations. The weak D type 38 was found in 1.5% of Caucasians with the Ccee phenotype,4 while a relatively higher frequency of 2.6% was recently detected among Brazilians with the Ccee phenotype.6 Although the RHD*weak D type 38 is considered a very rare allele, it is relatively common in the Portuguese population.7 In this study we analyzed three generations of a family with RHD*weak D type 38 using serologic and molecular methods.
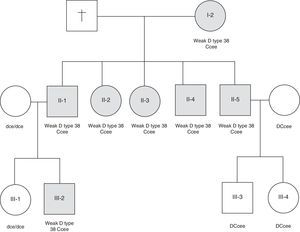
Case reportFamily study and blood samplesA sample from a 45-year-old female blood donor was tested D negative by routine serologic tests (Ccee phenotype) but positive by the adsorption-elution technique. In order to elucidate these results we examined blood samples from 12 members of her family were examined.
Immunohematologic serological tests were performed using commercial monoclonal antibodies (MoAbs) MS-26+TH-28 and ESD1 by classical serology techniques, hemagglutination in gel cards (DiaMed, Latino América S.A.) and by the tube methods with anti-D MoAbs IgM (HM10, P3X61, P3X21211F1, P3X21223B10; Diagast, Loos, France) and anti-D MoAbs IgG using the indirect antiglobulin test (IAT) (HM16, P3X35, P3X241, P3X249, P3X290; Diagast, Loos, France). Rh phenotypes were performed by hemagglutination in gel cards, according to the manufacturer's instructions (DiaMed, Latino América S.A.) using polyclonal antibodies against D, C, c, E and e.
The D antigen density of samples was determined by flow cytometry (FACSCalibur, Becton Dickinson, Heidelberg, Germany) according to a previously described protocol8 with seven anti-D IgG MoAbs, HM16, P3X35, P3X241,P3X249, P3X290 (Diagast, Loos, France) and MS26 and ESD1 (DiaMed Latino América S.A.).
Blood samples from family members were investigated by polymerase chain reaction (PCR) with specific primers for two genomic regions of the RHD gene, intron 4 and exon 10 and all ten RHD exons were sequenced full length.9,10
We found seven members of this family tested D negative by routine serologic tests (Ccee phenotype) and positive by the adsorption–elution technique. Their RBCs reacted negatively to four IgM anti-D, but showed weakly positive results with four IgG anti-D reagents (P3X249, P3X35, P3X241, HM16) (Table 1). The flow cytometric analysis of samples from these seven subjects showed D antigen densities ranging from 60 to 80 sites per cell. Molecular analysis identified the presence of RHD*weak D type 38 in three generations of this Brazilian family (Figure 1).
Pattern of reactivity seen with different clones of anti-D antibodies and D antigen densities tested against red blood cells from individuals with RHD*weak D type 38.
| Anti-D MoAb | D epitope | Isotype | Reactivity |
|---|---|---|---|
| P3X249 | 2.1 | G | (±) |
| P3X290 | 3.1 | G | (−) |
| ESD1 | 4.1 | G | (−) |
| P3X35 | 5.4 | G | W |
| P3X241 | 5.4 | G | (±) |
| HM16 | 6.4 | G | W |
| P3X61 | 6.4 | M | (−) |
| HM10 | 6.6 | M | (−) |
| P3X21211F1 | 8.2 | M | (−) |
| P3X21223B10 | 9.1 | M | (−) |
| MS26 | 9.1 | G | (−) |
| D Antigen densitiesa | 60–80 sites/cell | ||
MoAb: monoclonal antibody; (−): negative result; W: weak result; (±): very weak result.
This study revealed maternal inheritance (first generation) of the RHD*weak D type 38 with the Ccee phenotype by her five children and the second generation showed paternal inheritance by a son. This family evaluation showed that RHD*weak D type 38 was inherited as an ancestral allele.
In conclusion, this study found a relatively high prevalence of RHD*weak D type 38 in Brazilians, remembering that Brazil was colonized by the Portuguese.6,7 This is the first report showing the antigen densities of this weak D type with different MoAbs. The low D density found (60–80 sites/cell) could explain why weak D type 38 subjects are routinely mistyped as D negative by standard serologic methods including IAT. Thus, this could represent a potential risk in blood transfusion since units of blood collected from these individuals could be transfused to D-negative recipients causing RhD alloimmunization.
Conflicts of interestThe authors declare no conflicts of interest.