The use of high-dose chemotherapy with autologous support of hematopoietic progenitor cells is an effective strategy to treat various hematologic neoplasms, such as non-Hodgkin lymphomas and multiple myeloma. Mobilized peripheral blood progenitor cells are the main source of support for autologous transplants, and collection of an adequate number of hematopoietic progenitor cells is a critical step in the autologous transplant procedure. Traditional strategies, based on the use of growth factors with or without chemotherapy, have limitations even when remobilizations are performed. Granulocyte colony-stimulating factor is the most widely used agent for progenitor cell mobilization. The association of plerixafor, a C-X-C Chemokine receptor type 4 (CXCR4) inhibitor, to granulocyte colony stimulating factor generates rapid mobilization of hematopoietic progenitor cells. A literature review was performed of randomized studies comparing different mobilization schemes in the treatment of multiple myeloma and lymphomas to analyze their limitations and effectiveness in hematopoietic progenitor cell mobilization for autologous transplant. This analysis showed that the addition of plerixafor to granulocyte colony stimulating factor is well tolerated and results in a greater proportion of patients with non-Hodgkin lymphomas or multiple myeloma reaching optimal CD34+ cell collections with a smaller number of apheresis compared the use of granulocyte colony stimulating factor alone.
High-dose chemotherapy with autologous hematopoietic stem cell transplantation is an effective strategy to treat various hematologic neoplasms, such as chemosensitive relapsed Hodgkin's lymphomas,1,2 non-Hodgkin lymphomas (NHL)3,4 and multiple myeloma (MM).5 Several clinical guidelines and consensus recommend the procedure as standard treatment in these conditions.6–11 According to the Center for International Blood and Marrow Transplant Research (CIBMTR), 12,047 autologous hematopoietic stem cell transplantations (AHSCT) were carried out in the United States in 2011, with MM and NHL being the main indications.12 In Brazil, data from the Brazilian Transplant Registry show that 1144 AHSCT were performed in 2013, slightly higher than the year before.13
The CIBMTR shows that peripheral blood progenitor cells are the main source used to support autologous transplants.12 In addition to the possible chemoresistance of the cancer, mobilization of hematopoietic progenitor cells (HPC) is another potentially limiting step for AHSCT, with high failure rates (between 5% and 40%) associated with historically used mobilization strategies.14
A consensus published by the American Society for Blood and Marrow Transplantation (ASBMT) recommends collecting a minimum dose of 2×106CD34+ cells/kg to perform AHSCT, but the decision to accept collections of between 1×106 and 2×106CD34+ cells/kg can be individualized according to the circumstances of each patient. On the other hand, larger target numbers are needed if multiple transplants are planned.15
Although the minimum dose of progenitor cells to be collected is well defined, the ideal target or the desirable maximum dose is less clear. Some data show that the use of ≥5×106CD34+ cells/kg leads to quicker and more predictable grafting, achieving platelet transfusions independence significantly earlier with potential reductions in transplant costs.16,17 Thus, adequate progenitor cell mobilization is a key step when planning an AHSCT.
Biology related to mobilization of hematopoietic progenitor cells and therapeutic targetsAlthough mature hematopoietic cells are physiologically released from the bone marrow to the peripheral blood, immature cells are found in the circulation at a very low frequency. About 0.05% or less of the total circulating leukocytes are HPC and express the CD34+ surface marker.18 HPC adhere to the bone marrow microenvironment by a variety of adhesive interactions.19 Furthermore, they express a wide range of surface receptors, such as adhesion molecules associated with angiopoietin-1 lymphocytes, very late antigen 4 (VLA4), and Mac-1, C-X-C chemokine receptors type 4 (CXCR4) and type 2 (CXCR2), the surface glycoproteins CD44 and CD62L, and tyrosine kinase receptor c-kit.19 The bone marrow stroma contains stromal cell-derived factor 1 (SDF-1), CXC chemokine GRO-β, vascular cell adhesion molecule (VCAM-1), KIT-ligand, P-selectin glycoprotein ligand and hyaluronic acid, all of which are ligands for the stem cell adhesion molecules.20 Preclinical data show that inhibition of these receptor–ligand interactions results in increased mobilization of progenitor cells.19–21
Growth factors [granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF)] are the most widely used agents for progenitor cell mobilization; they have two main mechanisms of action. The first is the production of proteases by hyperplastic myelomonocytic series, which induces the cleavage of SDF-1 by preventing its binding to CXCR4. The most studied protease is matrix metallopeptidase 9 (MMP-9), although dipeptidase CD26 seems to have a greater role in this process.22,23 The second main mechanism is also proteolysis induced and is responsible for degradation of VCAM-1, osteopontin and fibronectin, leading to reduced adhesion of progenitor cells through its VLA-4 receptor in bone marrow stroma.23
The addition of a chemotherapeutic agent to a cytokine in the mobilization regimen has effects which are not fully elucidated.19 It is speculated that the addition of cyclophosphamide to growth factors has a synergistic effect on the release of granulocytic proteases in the bone marrow as its administration in isolation leads to cleavage of SDF-1, CXCR4 and c-kit adhesion molecules.19 Furthermore, the toxicity of chemotherapeutic agents on bone marrow stroma can release HPC as a result of damage to the functional ability of stromal cells in supporting them.19
Plerixafor (AMD3100) is a reversibly bicyclam inhibitor of CXCR4 that breaks the binding between SDF-1 and CXCR4 receptors, blocking the chemotactic signaling with stromal cells.23 Among the hypotheses for its mobilization mechanism is the loss of sensitivity of progenitor cells to SDF-1 caused by the inhibition of CXCR4. Consequently, these cells are attracted to the circulation through signaling probably related to sphingosine-1-phosphate (S1P), a sphingolipid implicated in the chemotaxis control of progenitor cells from bone marrow, blood and other tissues.23 Studies also suggest that plerixafor keeps the progenitor cells in the circulation by binding to CXCR4, leading to a loss of chemoattraction to SDF-1, decreasing HPC homing, which also contributes to mobilization.24
Mobilization of hematopoietic progenitor cells for multiple myeloma and lymphoma: results of the historically most used strategies show limitationsTraditionally, the most widely used mobilization strategies have been the use of growth factors alone (G-CSF/GM-CSF) or in combination with chemotherapeutic agents. Among the available growth factors, the most commonly used is recombinant G-CSF filgrastim, while others, such as, G-CSF pegfilgrastim, G-CSF lenograstim and GM-CSF molgramostim, are used less frequently.14
G-CSF alone as first-line mobilization is an attractive option owing to the predictable mobilization kinetics, which in turn allows predictable apheresis scheduling and staffing while decreasing costs of growth factors and the collection procedure compared with cyclophosphamide (CY).25–27
GM-CSF has been shown to be inferior to G-CSF in terms of number of stem cells collected and in post-transplantation outcomes related to hematopoietic recovery, transfusion and antibiotic support, febrile episodes and hospitalizations.28,29 It is most often used in remobilization strategies, alone or in combination with other cytokines or chemotherapy.28,29 Data on the use of pegfilgrastim in steady-state mobilization are both limited and mixed, but one study demonstrated predictable mobilization kinetics and similar collection yields and apheresis days compared with a separate G-CSF cohort.30
CY may be incorporated into the initial induction or salvage therapy cycles, or may be administered as a standalone cycle separately from standard therapy. The most common stand-alone regimens include cyclophosphamide at a range of doses between 2 and 7g/m2. CY is associated with higher cell yields, lower or similar failure rates,25–27,31–34 and improved engraftment kinetics,35–40 but also may result in more toxicity, febrile neutropenia, transfusions, hospitalizations and higher costs.14,34,41 The published literature on the various CY approaches is vast. In general, studies demonstrate that CY will mobilize more stem cells than G-CSF alone and help in the role of mobilizing traditionally difficult patients, such as those with lymphoma.42,43
The primary goal of mobilization is to collect a sufficient number of progenitor cells for the patient to undergo AHSCT. The optimal mobilization, however, requires collection of a target number of cells and also strategies to minimize the time and number of apheresis, reducing the cost of the procedure and avoiding complications related to mobilization, such as hospitalization due to febrile neutropenia.15
A systematic review of randomized clinical trials evaluated the results of 28 studies comparing different mobilization schemes. Eighteen included only patients with MM and/or lymphomas14 and only four of these were multicenter. Table 1 shows the characteristics of interventions and results observed in studies included in this systematic review that recruited ≥10 patients in each arm and compared different regimens of growth factors with and without chemotherapy (Table 1).
Randomized clinical trials evaluating different mobilization strategies including growth factors with/without chemotherapy.14
| Study | Interventions (n) | Collected CD34+ cells (median/×106) | Number of apheresis (median) | Patients failed to mobilize (%) |
|---|---|---|---|---|
| Cyclophosphamide associated with growth factor | ||||
| Facon et al., 1999 | CY 4g/m2+filgrastim 5mcg/kg/d+ancestim 20mcg/kg/d (n=55) | 12.4a | 1a | 14.5 |
| CY 4g/m2+filgrastim 5mcg/kg/d (n=47) | 8.2 | 2 | 19.1 | |
| Gazitt et al. 2000 | CY 3g/m2+molgramostim 250 mcg/m2 (n=10) | NR | 2 | 40.0 |
| CY 3g/m2+filgrastim 10mcg/kg/d (n=13) | NR | 3 | 23.0 | |
| CY 3g/m2+molgramostim 250 mcg/m2+filgrastim 10mcg/kg/d (n=12) | NR | 1 | 41.7 | |
| Narayanasami et al. 2001 | Filgrastim 10mcg/kg/d (n=23) | 2.5 | No difference | NR |
| CY 5g/m2+filgrastim 10mcg/kg/d (n=24) | 7.2* | No difference | NR | |
| Pavone et al. 2002 | DHAP+filgrastim 5mcg/kg/d (n=38) | 5.9 | 2 | 13.2 |
| CY+filgrastim 5mcg/kg/d (n=34) | 7.1 | 2 | 11.8 | |
| Vela-Ojeda et al. 2000 | IFO+molgramostim 5mcg/kg/d (n=28) | 3.1 | 3 | 14.3 |
| CY 4g/m2+molgramostim 5mcg/kg/d (n=28) | 5.3 | 3 | 10.7 | |
| Growth factor alone | ||||
| Stiff et al. 2000 | Filgrastim 10mcg/kg/d (n=51) | 3.6 | NR | 26.0 |
| Filgrastim 10mcg/kg/d+ancestim 20mcg/kg/d (n=56) | 2.4 | NR | 16.4 | |
| Other chemotherapy combinations associated with growth factors | ||||
| Copelan et al. 2009 | R+VP-16+filgrastim 10mcg/kg/d (n=28) | 9.9* | 3 | 3.6 |
| VP-16+filgrastim 10mcg/kg/d (n=27) | 5.6 | 4 | 14.8 | |
| Hart et al. 2009 | IEE+filgrastim 5 mcg/kg b.i.d+EPO (n=14) | 15.4 | 1.3 | 0 |
| IEE+filgrastim 5 mcg/kg b.i.d (n=12) | 12.6 | 1.8 | 16.7 | |
| Ozcelik et al. 2009 | CE+filgrastim 10mcg/kg/d (late) (n=23) | 10.8 | 1 | 13.0 |
| CE+filgrastim 10mcg/kg/d (early) (n=25) | 10.5 | 1 | 16.0 | |
CY: cyclophosphamide; IFO: ifosfamide; DHAP: dexamethasone+cytarabine+cisplatin; IEE: ifosfamide+epirubicin+etoposide; EPO: erythropoietin; CE: cyclophosphamide+etoposide; NR: not reported.
These studies show that, in general, strategies with higher doses of G-CSF alone or chemotherapy with the addition of growth factors (G-CSF and/or GM-CSF) result in increased number of CD34+ cells collected. Furthermore, some strategies reduced the number of apheresis needed to achieve target collection.14
Although this systematic review has limitations such as restricted sample size of studies, their moderate quality and variability in the population, it demonstrates the limitations of techniques historically used in HPC mobilization. A major retrospective analysis, involving 2177 patients undergoing attempted mobilization in three Italian centers between 1999 and 2007, showed a rate of poor mobilizers (defined as a collection of <2×106CD34+ cells/kg) of only 15% corroborating data from randomized trials.44
Insufficient initial mobilization leads to new mobilization procedures (remobilization), which can negatively influence disease progression and significantly increase the use of resources.41 Several studies evaluated the success of remobilization with traditional measures based on the use of growth factors. A retrospective analysis assessed the results of remobilization of regimens containing G-CSF and/or GM-CSF, alone or combined with chemotherapy in 251 patients with lymphoma or MM.41 After remobilization, only 18.4% of patients who used G-CSF or GM-CSF and 26.5% of those who received a growth factor associated with chemotherapy achieved collections of ≥2×106CD34+ cells/kg. When cells collected in the first mobilization were pooled, the failure rates were 28.1% in the group remobilized with growth factor alone and 47.1% in the group remobilized with growth factor associated with chemotherapy (Table 2).41
Results obtained with remobilization with G-CSF and/or GM-CSF with or without chemotherapy.
| Study and disease | Remobilization regimen (n) | Mobilized CD34+ cells (median/failure rate) | Apheresis days (median) | Apheresis days considering all mobilizations (median) | Failure rate considering all mobilizations after cells pooled (%) |
|---|---|---|---|---|---|
| Pusic et al., 200841 – lymphomas (n=NR) – multiple myeloma (n=NR) | G-CSF/GM-CSF (n=217) | 1.2×106/81.6% | 3 | 6 | 28.1 |
| G/C (n=34) | 0.9×106/73.5% | 2 | 6 | 47.1 | |
| Lefrere et al. 200445: – lymphomas (n=64) – myeloma (n=28) – acute leukemia (n=27) – solid tumors (n=19) | G-CSF+chemotherapy (cyclophosphamide in 57%) (n=138) | 3.5×106/35% | 2 | NR | 22.5 |
| Boeve et al. 200446: – lymphoma – myeloma Other cancers | G-CSF in high doses or G-CSF+GM-CSF (n=86) | 2.1×106/65% for lymphoma 3.5% for myeloma 31.5% for other cancers | 3 | NR | 26.7 after 2 mobilizations and 15 after 3 mobilizations |
G/C: growth factor associated with chemotherapy; NR: not reported.
Published data suggest that, although remobilization with growth factors can rescue patients who failed the first mobilization, the proportion of patients not meeting the minimum collection of HPC required to perform AHSCT is high (around 30%), confirming the need to evaluate more effective mobilization strategies.
Challenges for the identification and prospective characterization of poor mobilizers after strategies based on the use of growth factorsThe possibility of predicting mobilization failure based on patient's characteristics in the early mobilization process is highly debatable.15 Each study proposes different definitions of poor mobilizers, limiting the uniformity of the characteristics that prospectively identify these patients.18
Some identifiable characteristics before mobilization have often been associated with a higher risk of failure of procedures in patients with lymphoproliferative diseases.29 Although some of these factors are unanimously pointed out in the literature as predictive of poor mobilization, such as the intensity of prior exposure to chemotherapy or radiation, the use of chemotherapeutic agents and failure in a previous attempt of mobilization others, such as patient age, have a more controversial relationship with poor mobilization.47
The Italian working group, Gruppo Italiano Trapianto di Midollo Osseo (GITMO), recently proposed a definition for ‘poor mobilizers’ for patients with lymphoma or myeloma.48 This definition came from a consensus of experts and considers the factors described in Table 3.
Definitions for patients with lymphoma or myeloma who have prediction or evidence of poor mobilization.
| Predicted poor mobilizer | At least one major criterion or two minor criteria: • Major criteria: ○ Failure of previous attempt to mobilize; ○ Prior radiotherapy involving large areas containing bone marrow; ○ Complete courses of prior therapy including melphalan, fludarabine or other therapies that potentially affect progenitor cell mobilization such as carmustine, dacarbazine, cladribine, lenalidomide, and platinum compounds. • Minor criteria: ○ At least two previous lines of cytotoxic therapy; ○ Refractory disease; ○ Lymphoma is a higher risk factor than myeloma ○ Extensive involvement of bone marrow infiltration by tumor cells; ○ Bone marrow cellularity <30% in mobilization; ○ Low platelet count before deployment. ○ Age >65 years. |
| Proved poor mobilizer | • Having received adequate mobilization (G-CSF doses ≥10mcg/kg if used alone or ≥5mcg/kg if used after chemotherapy) and present: ○ a peak of circulating CD34+ cells <20/μL on days 4–6 after the start of mobilization with G-CSF alone or within 20 days after chemotherapy with G-CSF OR • Collection of CD34+ cells <2×106/kg in up to three apheresis |
The concept of proved poor mobilizer based on the circulating CD34+ cell count after 4–6 days of mobilization is very relevant given the availability of preemptive strategies with potential for reducing risk of complications related to the necessity of a greater number of apheresis or higher need for remobilizations and possible reduced cost.
Use of plerixafor to mobilize hematopoietic progenitor cells: randomized clinical trials showing its effectivenessPlerixafor has proved to be an agent that generates rapid mobilization of HPC in murine models and subsequently in healthy human volunteers.49 The phase I and II studies attested the mobilizing effect and safety of the G-CSF plus plerixafor combination in patients with NHL and MM. The rapid and significant increase in white blood and CD34+ cell counts in peripheral blood, included patients whose failure to mobilize with G-CSF alone was proven.49–51 The combination guaranteed collections of ≥2×106CD34+ cells/kg in 95.9% of patients with 77.6% achieving a collection of ≥5×106CD34+ cells/kg (22.4% of patients achieved this dose after only one apheresis).
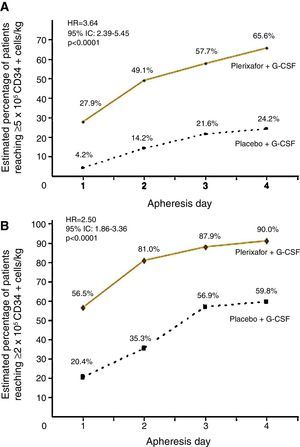
Two randomized, comparative, double-blind, placebo controlled and prospective multicenter studies evaluated the use of G-CSF plus plerixafor versus G-CSF alone in the initial mobilization strategy of patients. These studies recruited 298 patients with NHL52 and 302 patients with MM.53 NHL patients treated with the combined therapy obtained 65.6% of successful collections of ≥5×106CD34+ cells/kg versus 24.2% in the G-CSF arm. Collection of ≥2×106CD34+ cells/kg was achieved by 90% with the combination against 59.8% in the G-CSF group within four apheresis days (p-value<0.001) (Figure 1). The median number of CD34+ cells collected was also higher in combination group (5.69×106cells/kg versus 1.98×106cells/kg).52 Incidence of adverse events, mostly mild to moderate, was similar between groups with only 5.3% of serious events in the combination group and 6.9% in the G-CSF group during the mobilization period.52 The most common non-serious adverse events were diarrhea (38%), injection site erythema (29.3%), nausea (17.3%), headache (11.3%) and bone pain (10.7%), all of which were easily manageable.52
Percentage of patients with non-Hodgkin lymphoma achieving collection targets of ≥5×106CD34+ cells/kg (A) and of ≥2×106CD34+ cells/kg (B) after mobilization with G-CSF plus placebo or G-CSF plus plerixafor.
Adapted from DiPersio et al.52
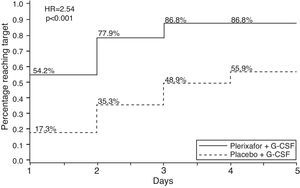
MM patients treated with G-CSF plus plerixafor obtained ≥6×106CD34+ cells/kg within two apheresis days, with 86.8% achieving this target versus 55.9% in the G-CSF group (p-value <0.0001). The combination was also more effective to collect ≥6×106CD34+ cells/kg within four apheresis days (75.7% vs. 51.3%; p-value <0.001) and ≥2×106CD34+ cells/kg days (95.3% vs. 88.3%; p-value=0.031)36 (Figure 2). The median number of CD34+ cells collected was also higher in the combination group (12.97 vs. 7.31×106 cells/kg). The incidence of adverse events was similar between study groups. The most common non-serious adverse events occurred were injection site erythema (20.4%), diarrhea (18.4%), nausea (16.3%), bone pain (9.5%) and fatigue (8.2%), all of which were easily manageable.53 The majority of these events occurred in the period after mobilization and were unrelated to the study drug.53
Percentage of patients with multiple myeloma achieving collection targets of ≥6×106CD34+ cells/kg after mobilization with G-CSF plus placebo or G-CSF plus plerixafor.
Adapted from DiPersio et al.53
These studies show that the combination of plerixafor with G-CSF is well tolerated and results in a greater proportion of NHL or MM patients achieving CD34+ cell collections considered optimal for transplant within a smaller number of apheresis days when compared with G-CSF alone.
Strategies for preemptive use of plerixaforMany studies, including phase III studies, have shown that the use of plerixafor reduces risk of poor progenitor cell mobilization and risk of failure from up to 40% to less than 10%. However, the cost of plerixafor may be a limiting factor for its unrestricted use in the initial mobilization.54
An alternative strategy discusses the use of plerixafor as rescue treatment in patients failing prior mobilization. Micallef et al. analyzed 52 patients with NHL who failed the initial mobilization with G-CSF in a phase III study. The analysis showed that patients who received remobilization with G-CSF associated with plerixafor presented a median collection of 2.9×106CD34+ cells/kg in remobilization after a median of three apheresis.55 In this study, 63.5% of patients achieved a collection of ≥2×106CD34+ cells/kg in up to four apheresis days in the second mobilization and 88.75% were able to undergo the transplant.
Some centers developed algorithms for the preemptive use of plerixafor in which patients initially receive customary regimens for mobilization. In this case, plerixafor is indicated, if necessary, to immediately rescue patients with verified poor mobilization before collection of progenitor cells.17,56
One study evaluated 314 patients with myeloma, amyloidosis or lymphoma undergoing mobilization with G-CSF and tested two algorithms for plerixafor use. One used plerixafor only in patients with a CD34+ cell count in peripheral blood of <10/μL on Day 4 of mobilization or on any day of apheresis, with collections of <0.5×106CD34+ cells/kg (plerixafor-1 algorithm – including 216 patients). The other used plerixafor if CD34+ cell count in peripheral blood reached <10/μL on Day 4 of mobilization (<20/μL if multiple transplants were planned) or if collection was <1.5×106CD34+ cells/kg on Day 1 of apheresis or <0.5×106CD34+ cells/kg in any subsequent apheresis day (plerixafor-2 algorithm – including 98 patients). The target was the collection of ≥4×106CD34+ cells/kg (optimal collection) or ≥2×106CD34+ cells/kg (minimum collection). The results were compared with those obtained in another cohort of 278 patients mobilized before the introduction of plerixafor, and with data from the same prospective database of transplant (Mayo Clinic), presenting similar characteristics.57 Some other studies evaluating different algorithms for the preemptive use of plerixafor showed lower collecting failure rates of 0–7%.57,58
A review of pharmacoeconomic studies presented some algorithms for the preemptive use of plerixafor that resulted in reduced consumption of several health resources, including the possibility of reducing the total cost of mobilization procedures.54
Cost effective analysesThere are some analyses related to plerixafor cost–benefit in front line and in remobilization scenarios,56,59–62 but they were not performed in Brazilian institutions nor did they analyze real private or public costs. Another important question about these analyses is that the calculated costs did not include supportive care costs, including transfusions, antimicrobials, hospitalization, cryopreservation, nursing and medical costs, patient's personal costs, logistics, or even costs with previous failures, remobilization56,59–62 and, specifically, the worse outcome in lymphoma and myeloma patients failure to undergo AHSCT.41,63,64,65
In the study of Micallef et al.,56 although the earlier identification of poor mobilizers and addition of plerixafor results in higher per-patient costs over baseline, it is associated with higher apheresis yields, fewer days of mobilization and collection, and lower failure rates. The median total costs of mobilization per patient, including any remobilization attempt, was US$12,500 in the baseline group, US$12,500 in the plerixafor-1 group, and US$20,000 in the plerixafor-2 group (Table 4). Although both the median and mean costs were higher in the plerixafor-2 group, the mobilization failure rate was 1%, with fewer days of apheresis, fewer days of plerixafor, and fewer total days of mobilization and collection compared to the plerixafor-1 algorithm.56 There is an urgency to have more accurate pharmacoeconomic matrixes to calculate the other important and neglected costs mentioned above and to decide about the real cost–benefit in each institution.
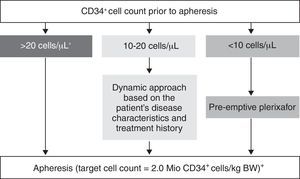
Consensus recommendations suggest the use of plerixafor as a mobilization strategy particularly in patients with a higher target for collection of progenitor cells, as a preemptive approach based on the monitoring of the CD34+ cell count and in cases of remobilization.15 The European Group for Blood and Marrow Transplantation (EGBMT) recommends preemptive use of plerixafor in patients with CD34+ cell counts <10/μL before apheresis31 (Figure 3). Each center should develop and implement its own algorithms for applying different mobilization strategies, with the goal of optimizing collection yields and costs–benefits.
European Group for Blood and Marrow Transplantation position regarding mobilization.
Adapted from Mohty et al.31
Collection of adequate numbers of HPC is a critical step in autologous transplantation procedures. Traditional strategies, based on the use of growth factors with or without chemotherapy, have some limitations even when remobilizations are performed.
The addition of plerixafor shows a consistent increase in collection rate success, reducing the number of apheresis and not increasing toxicity. Strategies of preemptive use of plerixafor have been considered a promising way to optimize and rationalize the use of this agent in patients who have high chance of failure with classic mobilization based on G-CSF with or without chemotherapy. This strategy would reduce failure in mobilization, especially in poor mobilizers, ensuring collection and transplantation as well as reducing time and costs of the mobilization procedure. However, external validity of these algorithms is limited, so it is recommended that each institution sets up a strategy appropriate to its standards for the preemptive use of plerixafor.
FundingPublication support provided by Sanofi.
Conflicts of interestDr. Marco Aurelio Salvino receives consulting and speaking fees. He is member of Sanofi Hematology Advisory Board.
Dr. Jefferson Ruiz works as hematology/oncology scientific advisor for Sanofi, Brazil.