The diagnosis of Multiple Myeloma is a challenge to the physician due to the non-specific symptoms (anemia, bone pain and recurrent infections) that are commonplace in the elderly population. However, early diagnosis is associated with less severe disease, including fewer patients presenting with acute renal injury, pathological fractures and severe anemia. Since 2006, the serum free light chain test Freelite® has been included alongside standard laboratory tests (serum and urine protein electrophoresis, and serum and urine immunofixation) as an aid in the identification of monoclonal proteins, which are a cornerstone for the diagnosis of Multiple Myeloma. The serum free light chain assay recognizes the light chain component of the immunoglobulin in its free form with high sensitivity. Other assays that measure light chains in the free and intact immunoglobulin forms are sensitive, but unfortunately, due to the nomenclature used, these assays (total light chains) are sometimes used in place of the free light chain assay. This paper reviews the available literature comparing the two assays and tries to clarify hypothetical limitations of the total assay to detect Multiple Myeloma. Furthermore, we elaborate on our study comparing the two assays used in 11 Light Chain Multiple Myeloma patients at presentation and 103 patients taken through the course of their disease. The aim of this article is to provide a clear discrimination between the two assays and to provide information to physicians and laboratory technicians so that they can utilize the International Myeloma Working Group guidelines.
Monoclonal Gammopathies (MGs) include premalignant Monoclonal Gammopathies of Uncertain Significance (MGUS), Smoldering/Indolent Multiple Myeloma and malignant [Solitary Plasmocytoma, Multiple Myeloma (MM), Light Chain Amyloidosis or Waldenstrom's Macroglobulinemia (WM)] conditions. These disorders are commonly characterized by the production of monoclonal proteins which may be either intact immunoglobulins (M-Ig), serum free light chains (sFLC), a combination of both, or rarely, free heavy chains only.1,2 A low percentage of these disorders present without the production of any monoclonal protein.
The asymptomatic disorders are identified through routine laboratory investigations, whilst the diagnosis of the symptomatic disorders can present considerable difficulties to the physician as the symptoms (anemia, recurrent infections, fatigue and bone pain) are common in elderly populations and are not specific to the disease.3–5 However, there is a need for timely diagnosis as delays can lead to an increased severity of the disease, including acute renal failure and pathological fractures, which can result in a shorter overall survival.6
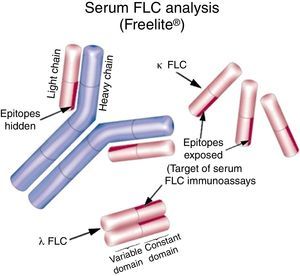
Immunoglobulin structure and sequence variationImmunoglobulins are the soluble, secreted form of the B-cell receptor and are composed of repeating mirror images comprising two identical heavy chains (gamma – γ, alpha – α, mu – μ, delta – δ or epsilon – ¿) and two identical light chains (kappa – κ or lambda – λ). Immunoglobulin heavy and light chains each have constant and variable regions. A pair of heavy and light chain variable regions together forms the antigen-binding site. The variable regions exhibit enormous structural diversity, particularly of antigen-binding contacts, allowing the recognition of a huge variety of antigens.
In humans, it is calculated that there are at least 1011 possible antibody structural variants, which allows for the recognition of a vast number of different antigens.7 The diversity is generated in four main ways.
Firstly, different combinations of gene segments are used in the rearrangement of heavy and light chain genes during early B-cell development. Kappa light chains are constructed from one of approximately 40 functional variable (Vκ) gene segments, one of 5 joining (Jκ) gene segments and a single constant (Cκ) gene. Lambda light chains are constructed from one of approximately 30 variable (Vλ) gene segments, and one of four (or more) pairs of functional joining (Jλ) gene segments and constant (Cλ) genes.7 The heavy chain variable region is formed from one of around 60 variable (VH), one of 30 diversity (DH), and one of six joining (JH) gene segments.7 This combinational diversity accounts for a substantial amount of variable region diversity. Secondly, diversity arises from the addition or removal of nucleotides at the junctions between V (D) and J gene segments during recombination. A third source of diversity arises from the many different combinations of heavy and light chains, and finally, somatic hypermutation introduces point mutations in the variable region genes of light and heavy chains in mature activated B-cells.7
In light chains, variations are also found in a region of the variable domain corresponding to the first 23 amino acids of the first framework region (a region not associated with antigen binding). Using monoclonal antibodies, four κ (Vκ I−Vκ IV) and six λ subgroups (Vλ I−Vλ VI) have been identified.8
Such diversity is best identified using polyclonal antibodies that can recognize an extensive range of different epitopes.
Introduction to Freelite®Freelite® (The Binding Site, UK) is the only nephelometric/turbidimetric assay cleared by the Food and Drug Administration (FDA) of the United States of America for the measurement of serum FLC (sFLC). It uses polyclonal antibodies produced in sheep that specifically recognize and quantify the kappa (κ) and lambda (λ) sFLC separately, enabling calculation of the kappa/lambda sFLC ratio (rFLC) which can be used to determine clonality.9,10 The antibodies specifically recognize epitopes present in the constant region of the light chains, which are hidden when joined to a heavy chain partner (i.e. in the form of the intact immunoglobulin) but are exposed when the light chains are in their free form (Figure 1). The sensitivity of assays has allowed quantification of normal circulating sFLC concentrations for the first time [Reference intervals: κ – median 7.3mg/L (95th percentile: 3.3–19.4mg/L); λ – median 12.4mg/L (95th percentile: 5.7–26.3mg/L); rFLC is 0.26–1.65].10,11 The majority of results of plasma cell dyscrasias show increased production of either the κ or λ sFLC. Individuals who have rFLC values >1.65 may have a monoclonal κ sFLC and those with rFLC values <0.26 may have a monoclonal λ FLC.12 The applicability of the rFLC in the clinical practice has been proven by a number of scientific publications which led to its inclusion in different international guidelines.13
Current techniques used for the detection of monoclonal proteinsSerum protein electrophoresis (SPEP) is routinely used to identify and quantify intact M-Ig, with immunofixation used to classify according to the heavy chain (γ, α, μ, δ and ¿) and light chain (κ or λ) isotypes.8 Whilst this technique is adequate for most, grossly elevated intact M-Igs, sensitivity can be limited due to co-migration and at low serum concentrations. Furthermore, SPEP poorly identifies sFLC14 meaning the assay is inadequate for the detection and quantitation of paraproteins produced in light chain MM or Amyloidosis.15 Historically, 24-hour urine collection has been recommended for the detection of immunoglobulin free light chains, however there is often poor compliance16–18 and renal function can heavily influence the accuracy of the results.19
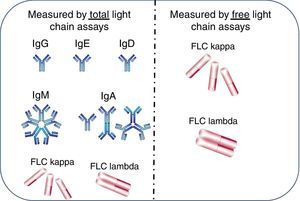
Differences between free light chain and total light chain assays (hypothetical)The use of the Freelite assays in the diagnosis of MGs has been well established.13 However there is often confusion between Freelite and similarly named assays which determine the total light chain concentration in serum and urine. The total light chain assay measures the concentration of all antibodies and free light chains of a particular light chain class i.e. IgG-κ+IgA-κ+IgM-κ+IgD-κ+IgE-κ+free κ. Freelite measures only the free form of the light chain (free κ in our example – Figure 2). Due to the difference in specificity of the assays, total light chain assays identify the light chain component of intact immunoglobulins and free light chains in serum whereas Freelite recognizes only the free light chain component. Therefore, there is a large difference in sensitivity (Table 1) between the two assays. The presence of a polyclonal background prevents the total immunoglobulin assay from being able to distinguish clonality at <4g/L, whereas the Freelite assay can detect clonality at mg/L concentrations.
Measurement of kappa (κ) and lambda (λ) light chains in free and total assays. Total light chain assays measure light chains when bound to heavy chains in intact immunoglobulins plus free light chains (FLC). The free light chain assay measures only free light chains.
Reference intervals and lower limits of sensitivity of free light chain assays and total light chain assays in serum.
| Parameter | Kappa reference interval (mg/L) | Kappa sensitivity (mg/L) | Lambda reference interval (mg/L) | Lambda sensitivity (mg/L) |
|---|---|---|---|---|
| Free light chains (The Binding Site) | 3.3–19.4 | 0.3 | 5.7–26.3 | 0.4 |
| Total light chains (Beckman Coulter) | 6290–13500 | 111 | 3130–7230 | 300 |
| Total light chains (Roche) | 1380–3750 | 300 | 930–2420 | 300 |
Recently, Hungria et al.20 published a study comparing the sensitivity of the sFLC assays to the total light chain assays for samples obtained from 114 light chain MM (LCMM) patients taken through the course of their disease. In keeping with previous reports15,19,21–34 the FLC identified clonality in 11/11 samples at presentation and identified persistent disease in 80/103 samples taken throughout the course of the disease. In contrast, the total light chain assay identified only 2/11 samples at presentation and 25/103 samples taken throughout the course of the disease. Somewhat confusingly, the light chain isotype was misreported in 11 samples as the opposite light chain.
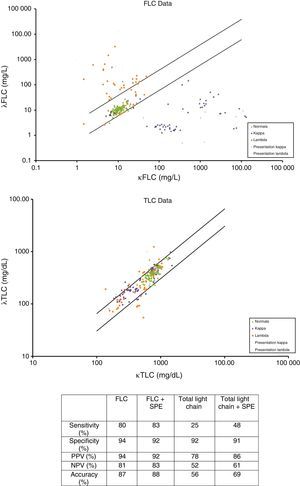
The International Myeloma Working Group (IMWG) guidelines for the identification of monoclonal immunoglobulins at presentation recommend an algorithm of Freelite+SPEP (Table 2). Hungria et al. showed that, in this study, 11/11 patients were identified using the Freelite assay and there was no need for SPEP. In contrast, total light chain+SPEP identified only 8/11 samples, clearly highlighting the lack of sensitivity of this algorithm recommended by the IMWG guidelines (Figure 3).
Scatter charts showing the differences in sensitivity between the Freelite® and total light chain assays for light chain myeloma patients taken at presentation (n=11) and through the course of their disease (n=103) compared to a 100 percentile normal range indicated by parallel lines. FLC: free light chain; SPE: serum protein electrophoresis; PPV: positive predictive value; NPV: negative predictive value.
The sensitivity of total κ/λ and sFLC assays were compared in a study by Marien et al.35 Sixteen serum samples from LCMM patients were investigated. Total κ and λ concentrations were measured using Beckman-Coulter reagents on the IMMAGE® nephelometer and sFLC concentrations were measured by Freelite assays (The Binding Site). All samples were abnormal by sFLC assays compared to only five of the 16 samples by total κ and λ assays. In addition, one λ patient was mistyped as κ by the total light chain assay. Other studies have confirmed that total light chain assays are less sensitive than sFLC analysis for the diagnosis of LCMM, Non-secretory MM and Amyloidosis.36–38
Summary of the importance of the serum free light chain assay screeningThe IMWG concluded that, for the purpose of screening for all MGs (with the exception of Amyloidosis), Freelite can replace 24-hour urine assessments.13 Furthermore, Katzmann et al.39 concluded that Freelite costs approximately half as much as 24-hour urine assessment based upon Medicare, USA 2006 reimbursement values.
Recently the IMWG updated the definition of MM to include additional, validated biomarkers alongside CRAB (hypercalcemia, renal failure, anemia, and bone lesions) assessments. A rFLC ≥100, with an involved free light chain concentration of >100mg/L was included in this validated biomarker list.40
Hematological responseInternational guidelines have included Freelite assessments as the most effective monitoring tool in patients with Amyloidosis.41,42 More recently, new response criteria were defined based upon changes in Freelite values during treatment.43 The response criteria utilized the rFLC and the difference between involved and uninvolved free light chains (dFLC); importantly the depth of the assigned response correlated to overall survival.
The IMWG recommend Freelite as the only available and reliable method for the determination of response in patients with Non-secretory and Oligosecretory MM.13 More recently in LCMM, comparisons of response assessment as determined by 24-hour urine evaluation and Freelite have suggested that Freelite is far superior to the 24-hour urine exam as a tool to measure patient response (Table 2).44
In all MM patients, normalization of the Freelite ratio corresponds to superior outcome independently of overall response.45 The IMWG have refined the definition of complete response, (i) negative immunofixation in serum and urine, (ii) disappearance of plasmacytomas, and (iii) bone marrow infiltration of plasma cells below 5%, to include a normal Freelite ratio.46 The new response definition, stringent complete response (sCR) relies on a normal Freelite ratio and negative plasma cell evaluation, and corresponds to an improved overall survival.47
ConclusionsEarly detection of patients with MM is key to a reduction in comorbidities that can impact the quality and duration of life. The sFLC assay, but not the total light chain assay, is an important part of the routine laboratory test algorithm that contributes to the identification of patients with MGs, including MM. To date, there are two pivotal studies that highlight the limited utility of the total light chain assay in the detection of MGs. In the study presented by Hungria et al., the addition of total light chain to SPEP failed to identify all LCMM patients, highlighting an important insensitivity when utilizing this assay. Similar results were reported by Marien et al. The assays can be easily distinguished based upon their normal ranges and caution is urged to utilize the sFLC assay, but not the total light chain assay for the screening, diagnosis and hematological responses in MGs.
Conflicts of interestDr. Vania TM Hungria receives research grants and is a speaker for The Binding Site. The others authors declare no conflicts of interest.
The authors are thankful to The Binding Site UK, University of Birmingham, UK and Fundação Hemocentro da Santa Casa de São Paulo, SP for their support.