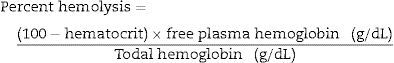Recent evidence shows a selective destruction of the youngest circulating red blood cells (neocytolysis) trigged by a drop in erythropoietin levels.
ObjectiveThe aim of this study was to evaluate the effect of recombinant human erythropoietin beta on the red blood cell storage lesion and apoptosis indices under blood bank conditions.
MethodsEach one of ten red blood cell units preserved in additive solution 5 was divided in two volumes of 100mL and assigned to one of two groups: erythropoietin (addition of 665IU of recombinant human erythropoietin) and control (isotonic buffer solution was added). The pharmacokinetic parameters of erythropoietin were estimated and the following parameters were measured weekly, for six weeks: Immunoreactive erythropoietin, hemolysis, percentage of non-discocytes, adenosine triphosphate, glucose, lactate, lactate dehydrogenase, and annexin-V/esterase activity. The t-test or Wilcoxon's test was used for statistical analysis with significance being set for a p-value <0.05.
ResultsErythropoietin, when added to red blood cell units, has a half-life >6 weeks under blood bank conditions, with persistent supernatant concentrations of erythropoietin during the entire storage period. Adenosine triphosphate was higher in the Erythropoietin Group in Week 6 (4.19±0.05μmol/L vs. 3.53±0.02μmol/L; p-value=0.009). The number of viable cells in the Erythropoietin Group was higher than in the Control Group (77%±3.8% vs. 71%±2.3%; p-value <0.05), while the number of apoptotic cells was lower (9.4%±0.3% vs. 22%±0.8%; p-value <0.05).
ConclusionsUnder standard blood bank conditions, an important proportion of red blood cells satisfy the criteria of apoptosis. Recombinant human erythropoietin beta seems to improve storage lesion parameters and mitigate apoptosis.
During storage under blood bank conditions, red blood cells (RBCs) undergo some deleterious changes that progressively affect their metabolism, cytoskeleton and membrane.1,2 Within certain limits, several substances added during storage improve and prolong the shelf life of RBCs by partially counteracting this ‘storage lesion’. However, the exact nature of this phenomenon and the precise combination of factors that ideally preserves RBCs are still unknown.3
Plasma removal during preparation suddenly places RBCs in a very different cellular environment that lasts the whole period of storage; in particular, the absence of erythropoietin (EPO), an essential cytokine that is associated to the viability, proliferation and maturation of RBC precursors.4 Some evidence shows that EPO not only maintains the viability of the immature erythroid component, but is also linked to the existence of mature RBC.5,6 In addition, mature RBCs can undergo a rapid self-destruction process with several features similar to apoptosis, including cell shrinkage, membrane microvesiculation, changes in shape, cytoskeleton alterations associated with protein degradation, and loss of cell membrane phospholipid asymmetry, leading to the externalization of phosphatidylserine.7,8
Now it is known that reticulocytes and young RBCs contain more EPO receptors than mature ones, suggesting that young RBCs are very sensitive to changes in plasmatic levels of EPO. Therefore, the number of EPO-binding sites may be a key factor in the selection and survival of different RBC populations. The greater number of EPO-binding sites on young RBCs could explain their preferential removal when EPO plasma levels drop below a certain threshold.9 Thus, a sudden decrease in EPO plasma concentration implies the selective destruction of the youngest circulating RBCs in a process named neocitolysis.10
A model of enucleated cells maintained under some easily accessible, manipulated and widely studied conditions, such as in blood banks, would allow us to establish a relationship between the phenomena of programmed cell death and the RBC storage lesion, in the absence or presence of their putative trophic factor, EPO. Therefore, if the storage lesion is a consequence, in part, of the activation of the apoptotic phenomena, the cytokine EPO could be a key factor that might reduce the occurrence of this event.
This study evaluates the effect of recombinant human EPO (rHuEPO) added to ten RBC units on some parameters measured during storage, including some apoptosis markers. From the point of view of the blood bank, a beneficial effect of rHuEPO will reflect as a longer shelf life of RBCs with better therapeutic benefits and fewer associated complications.
MethodsWhole blood (450mL±10%) was collected from ten volunteer donors in citrate phosphate dextrose adenine (CPDA) anticoagulant (63mL). After separation of the plasma and buffy coat by centrifugation, RBCs were suspended in 100mL of additive solution 5 (AS-5) and were 90% leukodepleted. Once prepared, each RBC unit was divided in two equal volumes: a dose of 665IU of recombinant human erythropoietin beta (rHuEPO) was added to one and 0.9% NaCl solution to the other as a control. The mixtures were stored under standard blood bank conditions (2–6°C). After gentle mixing in a laminar flow cabinet, 10mL samples were removed aseptically from each unit at baseline (Week 0 of storage) and every two weeks thereafter until Week 6 of storage.
The quantification of the extracellular rHuEPO concentration was by the enzyme-linked immunosorbent assay (ELISA) technique using a double antibody sandwich method (R&D Systems, Inc., Minneapolis). Free hemoglobin in supernatant was determined using the Hemocue Plasma/Low Hb technique. Hemolysis was calculated as the percentage of free extracellular hemoglobin and the total hemoglobin present in the sample. The formula used was:
Red cell morphology was evaluated by microscopy after diluting samples in Dacie's fluid; cells were counted as normal (discocytes) or anomalous (non-discocytes) and the proportion of anomalous cells is expressed as a percentage. The RBC adenosine triphosphate (ATP) concentration was estimated using an enzymatic ultraviolet protocol (Sigma, St. Louis, MO). Extracellular lactate, lactate dehydrogenase (LDH) and glucose were measured using standard clinical chemistry methods and reagents (Biosystems, Barcelona). All evaluations were made on Weeks 0, 2, 4 and 6 of storage.
At the end of storage (Day 42), externalization of phosphatidylserine on the RBC membrane and esterase activity were determined by 200 assays of annexin V and hydrolysis of 6-carboxyfluorescein diacetate (6-CFDA) by fluorescence microscopy (Sigma–Aldrich, St. Louis, Missouri). Briefly, the binding of annexin V to the cell membrane was visualized by means of the annexin V-Cy3.18 conjugate, in which, on using a green excitation filter (wavelength: 550nm), the Cy3.18 functions as a fluorochrome (red fluorescence). The esterase activity was visualized by the hydrolysis of 6-CFDA that, once hydrolyzed, generates the fluorescein fluorochrome (green fluorescence) using a blue-violet excitation filter (wavelength: 460nm). Therefore, the following events can be seen with each cell observed under a microscope: (1) Single green fluorescence, indicating esterase activity, characteristic of viable cells. (2) Single red fluorescence in the membrane, indicating annexin V bound to necrotic cells, and (3) green intracellular fluorescence and red fluorescence in membrane characteristic of cells undergoing apoptosis.
StatisticsStatistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) computer software (v. 13.0). Data are shown as means±standard deviation (SD). The t-test or Wilcoxon test were used to compare the Control and rHuEPO Groups and between the readings taken at 2-week intervals. P-values <0.05 were considered statically significant.
ResultsErythropoietin pharmacokineticsPharmacokinetic data after adding rHuEPO to the RBC units are shown in Table 1. Samples were obtained from RBC units before and immediately after adding the rHuEPO and weekly thereafter for 6 weeks. The quantity of 665IU of rHuEPO was chosen to obtain 1000 times the physiological EPO concentration during RBC storage.
Pharmacokinetics of 665IU recombinant human erythropoietin added to red blood cell units under blood bank conditions.
| Parameter | Value |
|---|---|
| Maximum concentration Cmax. (mIU/mL) | 12,333.06 |
| Volume of distribution (Vd) (mL) | 54.08 |
| Area under curve (AUC) 0 to 6 weeks (IU/mL) | 324.42 |
| Half-life (T½) | >6 weeks |
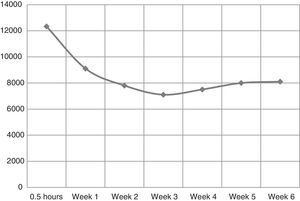
The EPO concentration before adding rHuEPO was 8.35mIU/mL. Half an hour after adding 665IU of rHuEPO, the mean concentration in the supernatant was 12.33IU/mL. By multiexponential kinetic analysis, initially there was a quick drop in the extracellular concentration of EPO followed by a progressive decrease with a tendency of stabilization at Week 2 (9.16IU/mL – 60% of the initial concentration). The concentration remained roughly constant until Week 6 (Figure 1).
Metabolic changes and red blood cell morphology during blood bank storageThe amount of hemolysis increased gradually during storage in both groups, without exceeding at any moment the maximum percentage allowed by the international quality standards (1%). There were no significant differences between the groups (p-value=0.953).
The percentage of non-discocytes increased progressively during storage in both rHuEPO and Control Groups from 0.1%±0.07 and 0.2%±0.04 at Week 0, to 14.3%±1.1 and 13.4%±1.3 at Week 6, respectively. There were no significant differences between the two groups at the end of the storage (p-value=0.172).
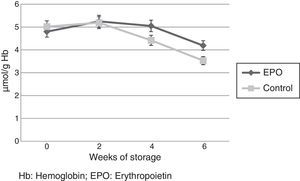
Red Cell ATP concentration (μmol/g Hb) decreased progressively in both groups during storage. However, the ATP concentration was significantly higher in the rHuEPO Group in Week 4 (5.05±0.1μmol/L vs. 4.42±0.08μmol/L; p-value=0.017) and Week 6 (4.19±0.05μmol/L vs. 3.53±0.02μmol/L; p-value=0.009) (Figure 2).
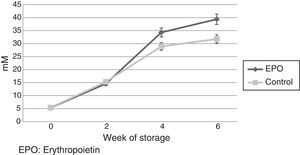
Extracellular lactate concentration increased progressively during the storage. It was significantly greater in the rHuEPO Group in Week 4 (34.29±1.7mmol/L vs. 28.92±1.3mmol/L; p-value=0.04) and Week 6 (39.38±1.9mmol/L vs. 31.76±1.1mmol/L; p-value=0.009) (Figure 3). Extracellular glucose concentration decreased progressively during storage without significant differences between the groups (p-value=0.646).
On the other hand, extracellular LDH activity increased progressively during storage in both groups and in Week 6, it was significantly higher in the rHuEPO Group (304±5.7IU/mL vs. 251±8.3IU/mL; p-value=0.05).
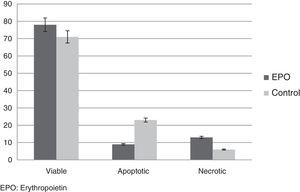
Apoptosis indicesPhosphatidylserine on the RBC membrane and the esterase activity were investigated by fluorescence microscopy at the end of storage (6th week). The fraction of cells with green fluorescence, indicating esterase activity and characteristic of cell viability, was significantly higher in the rHuEPO Group compared to the Control Group (77%±3.8% vs. 71%±2.3%; p-value <0.05). The fraction of cells with red fluorescence only (binding of annexin V), characteristic of necrotic cells, was significantly lower in the Control Group compared with the rHuEPO Group (6%±0.1% vs. 12%±0.6%; p-value <0.05). The cell fraction with green and red fluorescence, characteristic of cells undergoing apoptosis was lower in the units exposed to rHuEPO compared to the Control Group (9.4%±0.3% vs. 22%±0.8%; p-value <0.05) (Figure 4).
DiscussionNative EPO possesses multiple physiologic roles such as the stimulation of erythropoiesis, apoptosis rescue of astrocytes and neurons, bone regeneration, the stimulation of mitosis in cardiomyocytes and endothelial cells, megakaryocyte maturation, myeloid cell proliferation and tolerance of retina photoreceptors to hypoxia mediated by nitric oxide (NO), etc.11–13
The classic pattern of the actions of native EPO and rHuEPO related to erythroid line cells is linked to survival, proliferation and differentiation of committed stem cells [erythroid-burst forming units (UFB), erythroid-colony forming units (UFC)].13 However, some evidence14 indicates that these substances play a crucial role in the survival of mature circulating RBCs; most of this evidence is constituted in the context of the phenomenon of neocytolysis observed after an abrupt decrease in plasma levels of native or recombinant EPO.15 On the other hand, after the cessation of rHuEPO therapy, patients with anemia of chronic kidney disease experienced enhanced RBC death as soon as the EPO concentration declined.16
RBC units obtained and conserved under standard blood bank conditions are almost free of extracellular EPO due to the removal of blood plasma from the whole blood. Thus, it is possible that, what is known as ‘storage lesion’ is, at least in part, a reflection of the suppression of the effect of EPO on mature RBCs.
For the first time, this study shows the effects of the addition of rHuEPO to RBC units preserved under blood bank conditions. The results of this study are split into two categories:
- 1.
The pharmacokinetics of the addition of 665IU of rHuEPO to RBC units.
- 2.
RBC storage lesion parameters (under blood bank conditions) observed in the presence of rHuEPO.
The volume of immunoreactive rHuEPO (54.1mL/dL) is higher than the volume calculated (37.5mL/dL) according to the value of the hematocrit of RBC units (62.5%). Probably, rHuEPO moved toward a second compartment, in this case the RBC (adsorption by the cell membrane or entry into the intracellular compartment), or, it could be interpreted as a quick degradation of immunoreactive rHuEPO by proteases released from the remaining viable leukocytes in the RBC unit.
Both mechanisms are not mutually excluding. In the first case, declining rHuEPO concentrations follow a multiexponential behavior; stabilization is achieved by the second week of storage, in which the intracellular compartment would have been saturated or the proteases that are present in the extracellular medium would have lost their activity. In addition, from this week there is no decrease in the rHuEPO concentration, suggesting that its half-life is greater than six weeks. On the other hand, it is remarkable that rHuEPO did not have a deleterious effect on cell lysis parameters, hemolysis and LDH, which always remained below the maximum permitted limit according to international standards.17
It has been extensively documented that EPO acts on RBC progenitors by binding to a specific receptor (EPOr) located on the membrane, thus activating intracellular mechanisms that impede apoptosis and maintain red cell line proliferation and differentiation.18 The expression of EPOr during late erythroid development decreases exponentially, and it is claimed that mature RBCs virtually have no EPOr and are EPO insensitive. However, specific binding of EPO to human RBCs has been reported,19 and some direct effects on mature RBCs have been described such as glucose flux,20 antioxidant defense,21 and ion transportation.22
Previous research using 125I-EPO to track EPO binding to human RBCs reported the presence of five to six EPO binding sites per cell, based on the assumption that the number of specific EPO binding sites is equal for each RBC.22 The marked decrease in EPO-binding capability with cell aging is most likely achieved as the EPO receptor is released in vesicles during cell maturation or undergoes internalization and proteasomal degradation.23
Some evidence has shown the presence of a single class of EPO binding sites, similar in affinity and downstream targets to classic EPOr. EPO treatment results in upregulation of RBC NO production via activation of the PI3K/Akt signaling pathway and phosphorylation of RBC eNOS at Ser-1177.24 Additionally, there is evidence of a direct link between the EPO-induced regulation of RBC eNOS activity and the maintenance of the redox state in mouse RBCs. The increase in RBC-derived NO would reduce the scavenging of NO by endothelial cells, thereby facilitating NO-induced vasodilatation.25
With proteases in the extracellular medium, it is important to remember that the pH of the medium is slightly acidic (approximately 6.5),26 and consequently the proteolytic action on EPO is reduced. Additionally, rHuEPO is a peptide, which could diminish the access of proteases. In fact, in vivo, the highly glycosylated rHuEPO has a longer half-life than native EPO.27 On the other hand, unless proteolysis of the sequence of epitopes recognized by the antibodies used in the ELISA technique occurs, immunoreactive rHuEPO will not be detected.
Intracellular concentrations of ATP had a similar behavior in both groups (rHuEPO and control). However, in Weeks 4 and 6 of storage, the RBC ATP concentration was significantly higher in the rHuEPO Group than the Control Group. This result suggests that rHuEPO, through its receptor, is able to modify the energy metabolism of RBCs. In addition, rHuEPO diminishes the magnitude of one of the key indicators of storage lesion, ATP depletion.28 This smaller drop in intracellular ATP can be explained in two ways: first, rHuEPO diminishes the consumption of ATP and second, at 4°C this cytokine stimulates ATP production, or both. RBC ATP concentrations maintain cell viability and normal flow characteristics, causing local vasodilatation in the microcirculation.
The percentage of hemolysis was statistically similar in the two groups. This increase did not exceed the international quality standards (<1%) at any time. Hence, rHuEPO did not cause cell destruction. However, it cannot be assumed that similar proportions of hemolysis means similar mechanisms of destruction: apoptosis or necrosis (see below).
The extracellular lactate concentration progressively increased in both groups over the period of storage. Therefore, even at 4°C, glucose is depleted by the glycolytic pathway. However, in Weeks 4 and 6 of storage, lactate concentration was significantly higher in the rHuEPO Group, which means that during the storage period there was a significantly higher production of lactate in this group. This can be interpreted in two ways: first, there is a higher availability of the substrate (glucose) for the glycolytic pathway in the units with rHuEPO; second, glucose consumption in the glycolytic pathway is more efficient in the presence of rHuEPO as reflected in a higher lactate production. Then, rHuEPO is able to avoid, in part, the deterioration in the glycolytic pathway, which is the only physiologic mechanism that mature RBCs possess to produce ATP.
There was a progressive increase in LDH activity during storage in both groups. However, in Week 6, this activity was significantly higher in the rHuEPO Group. LDH leakage to the extracellular space can be explained in two ways: first, a rise in hemolysis in RBCs exposed to rHuEPO (this possibility was discarded because of the results of hemolysis). Second, as is discussed below, the percentage of necrotic cells in the rHuEPO Group was higher.
The annexin V and esterase activity showed that, under storage conditions at 4°C, rHuEPO is able to significantly improve cell viability; this fact is correlated to higher levels of ATP and lactate production. Additionally, under these conditions, treatment with rHuEPO decreased the proportion of cells that satisfy the two criteria for apoptosis by fluorescence microscopy, the appearance of phosphatidylserine on the RBC membrane and esterase activity.
Although the classic approaches of apoptosis defined for nucleated cells are not completely applicable to mature RBCs, a lot of evidence accumulated in the last few years indicates that mature RBCs are probably ‘mummified’, culminating a process of programmed death at the end of their physiologic period of life in the circulation.29 However, there is no previous published evidence about the course of this peculiar process of apoptosis or ‘eryptosis’ and the conditions of prolonged aging achieved during storage in blood bank conditions. Here, it is necessary to keep in mind that, at the end of 6 weeks of storage in AS-5, 1/120 of the cells have an age of almost 162 days, which is higher than the maximum age reached in vivo.7
This study shows for the first time that, under standard blood bank conditions, an important proportion of RBCs satisfy the criteria of apoptosis, indicating that the preservation conditions/solutions used, do not prevent this phenomenon, and that this is associated with the short lifespan of transfused RBCs. Therefore, it is interesting that the addition of rHuEPO can significantly reduce the proportion of RBC satisfying the characteristics for apoptosis, thus opening the discussion about the improved preservation of these cells in an EPO-enriched media.
However, the effects of rHuEPO were accompanied by a higher proportion of necrotic cells, which can be interpreted in two ways: first, rHuEPO may modulate the final behavior of RBCs, diverting their behavior from apoptosis to necrosis, or second, rHuEPO induces cell necrosis. This latter event has not been documented in previous studies (in vivo or in vitro) in respect to different cell types using native EPO or rHuEPO. In spite of this fact, it is interesting that the number of viable cells was higher in the rHuEPO Group.
RBC apoptosis or eryptosis during blood bank storage can be a direct consequence of oxidative stress and caspase-mediated fragmentation involving the loss of band 3 protein thereby starting a process of immune recognition and phagocytic removal of senescent and impaired RBC from circulation. In addition, it was reported that EPO was able to protect the RBC membrane from oxidative damage (lipid peroxidation) by scavenging hydroxyl radicals. Indeed, EPO was able to inhibit the externalization of phosphatidylserine in a model of oxidative stress presumably via the EPOr.30
ConclusionsFuture studies are necessary to identify the minimum dose of EPO needed to achieve the desirable effects of increases in the proportion of viable cells and decreases in apoptotic cells, keeping in mind that, the increment in the proportion of viable cells is accompanied by a significant improvement of key parameters related to the RBC storage lesion.
The mechanisms involved in the death of circulating RBCs as well as those related to increased survival are of great interest because, apart from their association to the treatment of anemia, storage conditions in blood banks will be improved by increasing the shelf life and quality of stored RBCs, with better clinical benefits for patients.
Conflicts of interestThe authors declare no conflicts of interest.
We thank the Colombian Red Cross for providing the RBC units used in this study.