Multiple myeloma is a disease of the elderly. However, 40% of patients are diagnosed before 65 years old. Outcomes regarding age as a prognostic factor in MM are heterogeneous.
MethodWe retrospectively analyzed clinical characteristics, response to treatment and survival of 282 patients with active newly-diagnosed multiple myeloma, comparing results between patients younger and older than 65 years.
Main resultsThe frequency of multiple myeloma in those younger than 66 years was 53.2%. Younger patients presented with a more aggressive disease, more advanced Durie-Salmon stage (85.3% vs 73.5%; p=0.013), extramedullary disease (12.7% vs 0%; p<0.001), osteolytic lesions (78.7% vs 57.6%; p<0.001) and bone plasmacytoma (25.3% vs 11.4%; p=0.003). In spite of this, the overall response rate was similar between groups (80.6% vs 81.4%; p=0.866). The overall survival was significantly longer in young patients (median, 65 months vs 41 months; p=0.001) and higher in those who received autologous hematopoietic stem cell transplantation. The main cause of death was disease progression in both groups. Multivariable analysis revealed that creatinine ≥2mg/dl, extramedullary disease, ≤very good partial remission and non-autologous hematopoietic stem cell transplantation are independent risk factors for shorter survival.
ConclusionAlthough multiple myeloma patients younger than 66 years of age have an aggressive presentation, this did not translate into an inferior overall survival, particularly in those undergoing autologous hematopoietic stem cell transplantation.
Multiple myeloma (MM) represents 1% of all cancers and is the second most common hematologic malignancy, after lymphoma.1 MM is a disease of the elderly, with the median age at diagnosis of 66–70 years. However, 40% of patients are younger than 65 years and 10% younger than 50 years. It is uncommon in younger than 40 years and extremely rare before 30 years, with a reported frequency of 2% and less than 0.5%, respectively.2–4
Survival is variable, depending on the different prognostic factors, ranging from months to more than 10 years. Age is a prognostic factor observed in every kind of cancer. Publications regarding age as a prognostic factor in MM are heterogeneous.4–10
Most data regarding the impact of age on survival mainly come from clinical trials developed before the current therapy choices were in use. Information regarding outcomes in young patients, compared to older patients, treated with novel agents is limited, particularly in the real-world setting. The aims of the present study were to describe clinical features at diagnosis, response to treatment and survival in 282 patients with active newly-diagnosed MM (NDMM) and compare outcomes between patients younger and older than 65 years.
MethodsA retrospective, descriptive analysis of all consecutive active NDMM patients recorded in the Uruguayan MM Registry between January 2011 and December 2018 were included. Patients with monoclonal gammopathy of unknown significance, smoldering MM, plasma cell leukemia and amyloidosis were excluded.
Epidemiological and clinical data were collected from medical charts at each institution. Characteristics of the disease at diagnosis, first-line treatment and response, date of progression or death and cause of mortality were detailed. Causes of death were grouped as MM progression, infections and others, as defined by the treating physician. Date of death was obtained from the Uruguayan Ministry of Health database.
Patients ≤65 years old were considered “young”, while those >65 years are referred to as “old”. Diagnosis and response to therapy were defined according to the International Myeloma Working Group Criteria.9,10
Consent from the Ethics Committees to review all charts were obtained at each institution. Centers that did not provide consent were not included in this analysis.
Statistical analysisThe statistical analysis was performed using the statistical database SPSS (Statistical Package for the Social Science, version 25.0). Quantitative variables were described in terms of mean, median and standard deviation (SD); qualitative variables were described as absolute percentage for each modality. Subgroup comparisons were performed for exploratory analysis. Differences between subgroups were analyzed using the Chi-square test and Student's T-test, as appropriate. Survival curves were constructed according to the Kaplan–Meier method and curves were compared using the log-rank test. The Cox regression analysis was used to identify prognostic factors for survival in patients 65 years or under. In all cases, p<0.05 was considered significant.
ResultsDisease characteristicsIn the study period, 282 MM patients were diagnosed. The median age at diagnosis was 64.5 years (range 32–94 years); 150 (53.2%) patients were ≤65 years, 59 (20.1%) between 51 and 60 years and 39 (13.8%) between 41 and 50 years. Only 6 (2.1%) patients were ≤40 years and none were ≤30 years. The median age at diagnosis in young patients was 57 years (range 32–65 years).
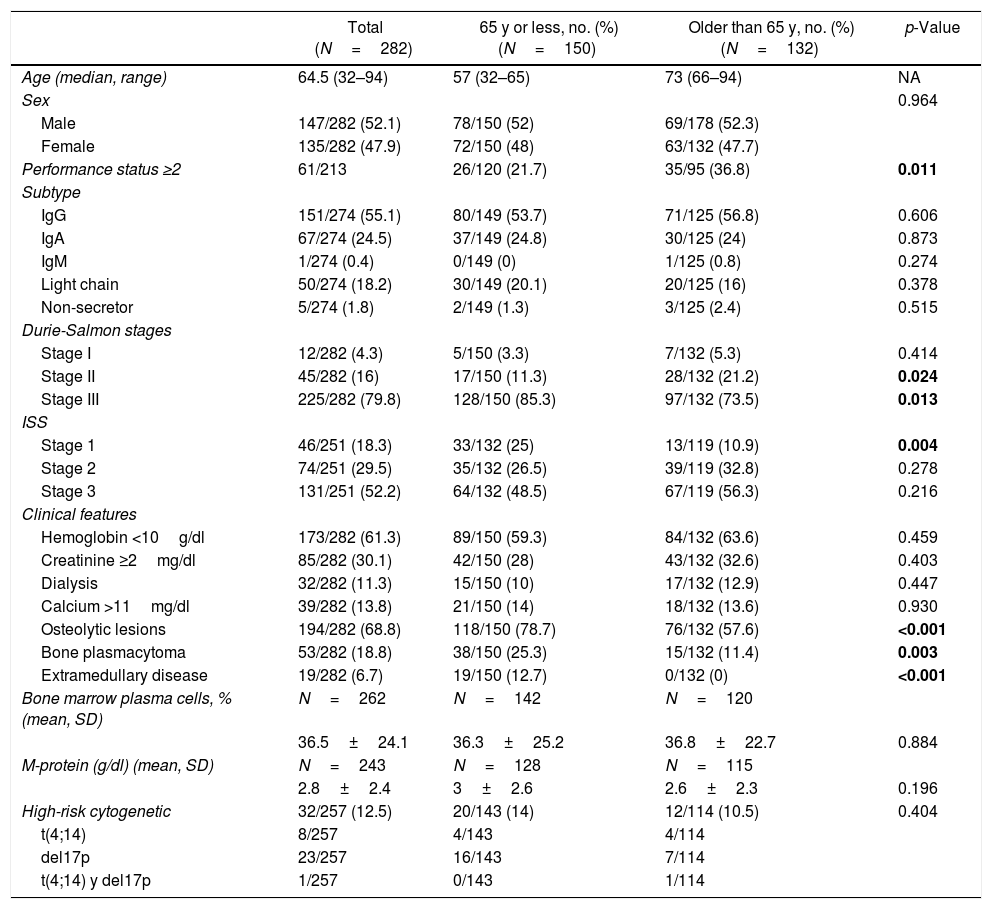
Characteristics at diagnosis of young and old patients are shown in Table 1. Advanced Durie-Salmon (DS) stage (stage III) was more frequent in young patients (85.3% vs 73.5%; p=0.013). Although both groups had a high international scoring system (ISS) score at diagnosis, ISS 1 was more frequent in young patients (25% vs 10.9%; p=0.004). The Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥2 was more frequent in older patients (36.8% vs 21.7%; p=0.011).
Characteristics of patients at diagnosis.
| Total (N=282) | 65 y or less, no. (%) (N=150) | Older than 65 y, no. (%) (N=132) | p-Value | |
|---|---|---|---|---|
| Age (median, range) | 64.5 (32–94) | 57 (32–65) | 73 (66–94) | NA |
| Sex | 0.964 | |||
| Male | 147/282 (52.1) | 78/150 (52) | 69/178 (52.3) | |
| Female | 135/282 (47.9) | 72/150 (48) | 63/132 (47.7) | |
| Performance status ≥2 | 61/213 | 26/120 (21.7) | 35/95 (36.8) | 0.011 |
| Subtype | ||||
| IgG | 151/274 (55.1) | 80/149 (53.7) | 71/125 (56.8) | 0.606 |
| IgA | 67/274 (24.5) | 37/149 (24.8) | 30/125 (24) | 0.873 |
| IgM | 1/274 (0.4) | 0/149 (0) | 1/125 (0.8) | 0.274 |
| Light chain | 50/274 (18.2) | 30/149 (20.1) | 20/125 (16) | 0.378 |
| Non-secretor | 5/274 (1.8) | 2/149 (1.3) | 3/125 (2.4) | 0.515 |
| Durie-Salmon stages | ||||
| Stage I | 12/282 (4.3) | 5/150 (3.3) | 7/132 (5.3) | 0.414 |
| Stage II | 45/282 (16) | 17/150 (11.3) | 28/132 (21.2) | 0.024 |
| Stage III | 225/282 (79.8) | 128/150 (85.3) | 97/132 (73.5) | 0.013 |
| ISS | ||||
| Stage 1 | 46/251 (18.3) | 33/132 (25) | 13/119 (10.9) | 0.004 |
| Stage 2 | 74/251 (29.5) | 35/132 (26.5) | 39/119 (32.8) | 0.278 |
| Stage 3 | 131/251 (52.2) | 64/132 (48.5) | 67/119 (56.3) | 0.216 |
| Clinical features | ||||
| Hemoglobin <10g/dl | 173/282 (61.3) | 89/150 (59.3) | 84/132 (63.6) | 0.459 |
| Creatinine ≥2mg/dl | 85/282 (30.1) | 42/150 (28) | 43/132 (32.6) | 0.403 |
| Dialysis | 32/282 (11.3) | 15/150 (10) | 17/132 (12.9) | 0.447 |
| Calcium >11mg/dl | 39/282 (13.8) | 21/150 (14) | 18/132 (13.6) | 0.930 |
| Osteolytic lesions | 194/282 (68.8) | 118/150 (78.7) | 76/132 (57.6) | <0.001 |
| Bone plasmacytoma | 53/282 (18.8) | 38/150 (25.3) | 15/132 (11.4) | 0.003 |
| Extramedullary disease | 19/282 (6.7) | 19/150 (12.7) | 0/132 (0) | <0.001 |
| Bone marrow plasma cells, % (mean, SD) | N=262 | N=142 | N=120 | |
| 36.5±24.1 | 36.3±25.2 | 36.8±22.7 | 0.884 | |
| M-protein (g/dl) (mean, SD) | N=243 | N=128 | N=115 | |
| 2.8±2.4 | 3±2.6 | 2.6±2.3 | 0.196 | |
| High-risk cytogenetic | 32/257 (12.5) | 20/143 (14) | 12/114 (10.5) | 0.404 |
| t(4;14) | 8/257 | 4/143 | 4/114 | |
| del17p | 23/257 | 16/143 | 7/114 | |
| t(4;14) y del17p | 1/257 | 0/143 | 1/114 |
The values in blod highlight that these variables had a significant difference (p<0.05).
The most common subtypes were immunoglobulin G (IgG), followed by IgA and light chain in both groups. The mean monoclonal protein spike, bone marrow (BM) plasmacytosis, low hemoglobin, increased serum creatinine and hypercalcemia did not differ significantly between the two groups. Osteolytic lesions (78.7% vs 57.6%; p<0.001), bone plasmacytoma (25.3% vs 11.4; p<0.003) and extramedullary disease (12.7% vs 0%; p<0.001) were more frequent in younger patients.
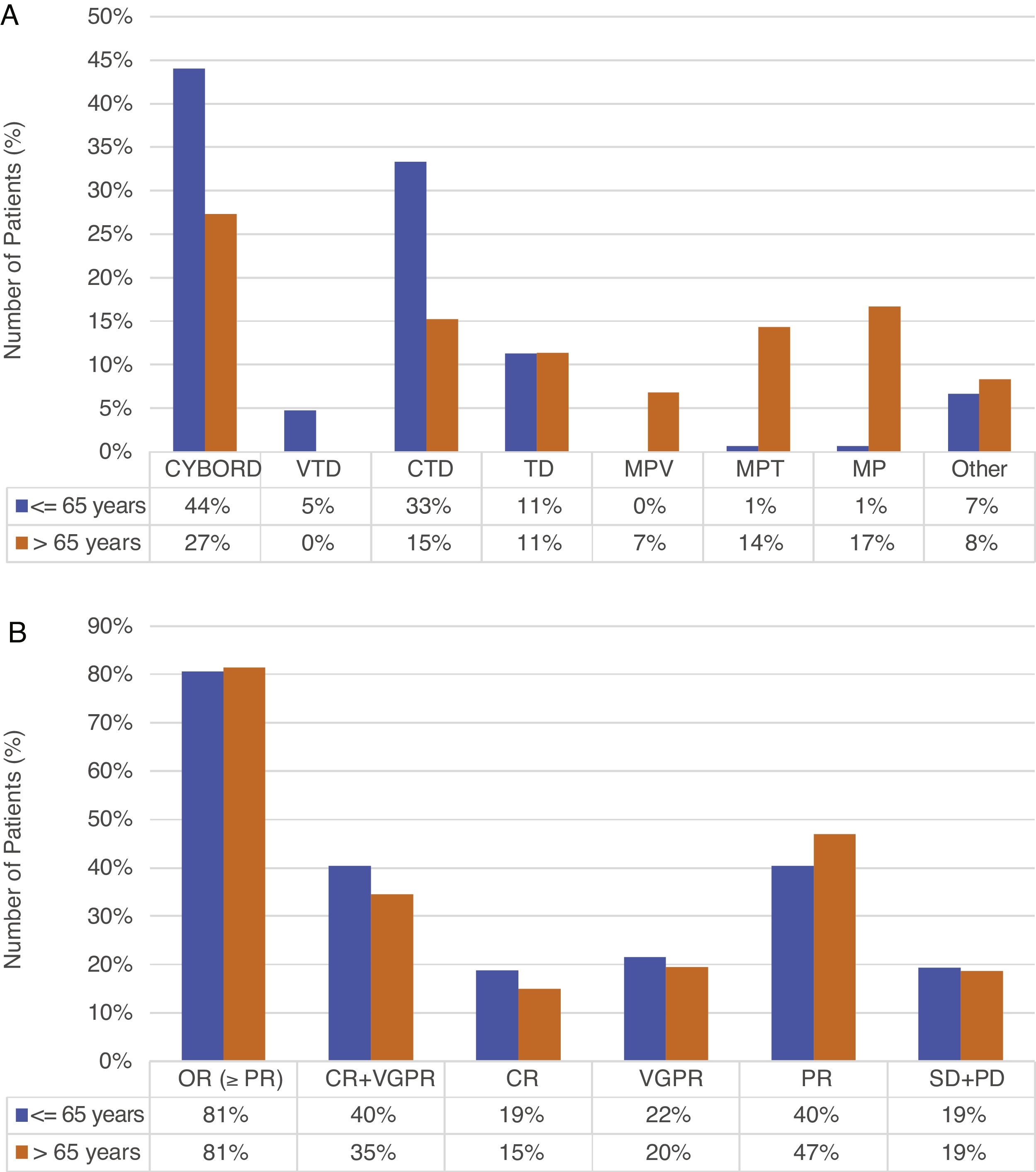
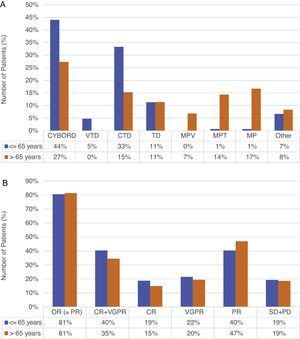
Treatment and responseAll patients received conventional therapy; in both groups, the main induction regimen was cyclophosphamide–bortezomib–dexamethasone (CyBorD), used in 44% (66/150) of the young vs 27.3% (36/132) of the old. The second regimens in frequency were cyclophosphamide–thalidomide–dexamethasone (CTD) and melphalan prednisone (MP) in young and old patients, respectively (33.3% and 16.7%). A total of 48% of the young patients received bortezomib-based therapy, compared to 34.1% of the older (p=0.018). Regimens administered are summarized in Figure 1(A).
Frist-line treatment (A) and response (B) in ≤5 and >65 years (n=282).
Abbreviations. CYBORD: cyclophosphamide+bortezomib+dexamethasone; VTD: bortezomib+thalidomide+dexamethasone; CTD: cyclophosphamide+thalidomide+dexamethasone; TD: thalidomide+dexamethasone; MPV: melphalan+prednisone+bortezomib; MPT: melphalan+prednisone+thalidomide; MP: melphalan+prednisone; VAD: vincristine+doxorubicin+dexamethasone; DT-PACE: dexamethasone+thalidomide+cisplatin, doxorubicin+cyclophosphamide+etoposide; OR: overall response; CR: complete response; VGPR: very good partial remission; PR: partial remission; SD: stable disease; PD: progressive disease.
Frontline consolidation with autologous stem cell transplant (ASCT) was performed in 54.7% of young patients, while only in 24% of patients between 66 and 70 years (p<0.001). No patients over 70 years received ASCT.
Overall response rates were similar in both groups (80.6% vs 81.4%; p=0.866). Although the difference was not significant, young patients achieved higher rates of complete remission (CR) (18.7% vs 15%; p=0.442) and very good partial remission (VGPR) (21.6% vs 19.5%; p=0.680), while partial remission (PR) was higher in older patients (40.3% vs 46.9%; p=0.292). Responses are summarized in Figure 1(B). Maintenance treatment was indicated in 23.3% of the young, versus 8.3% of the old patients (p<0.001).
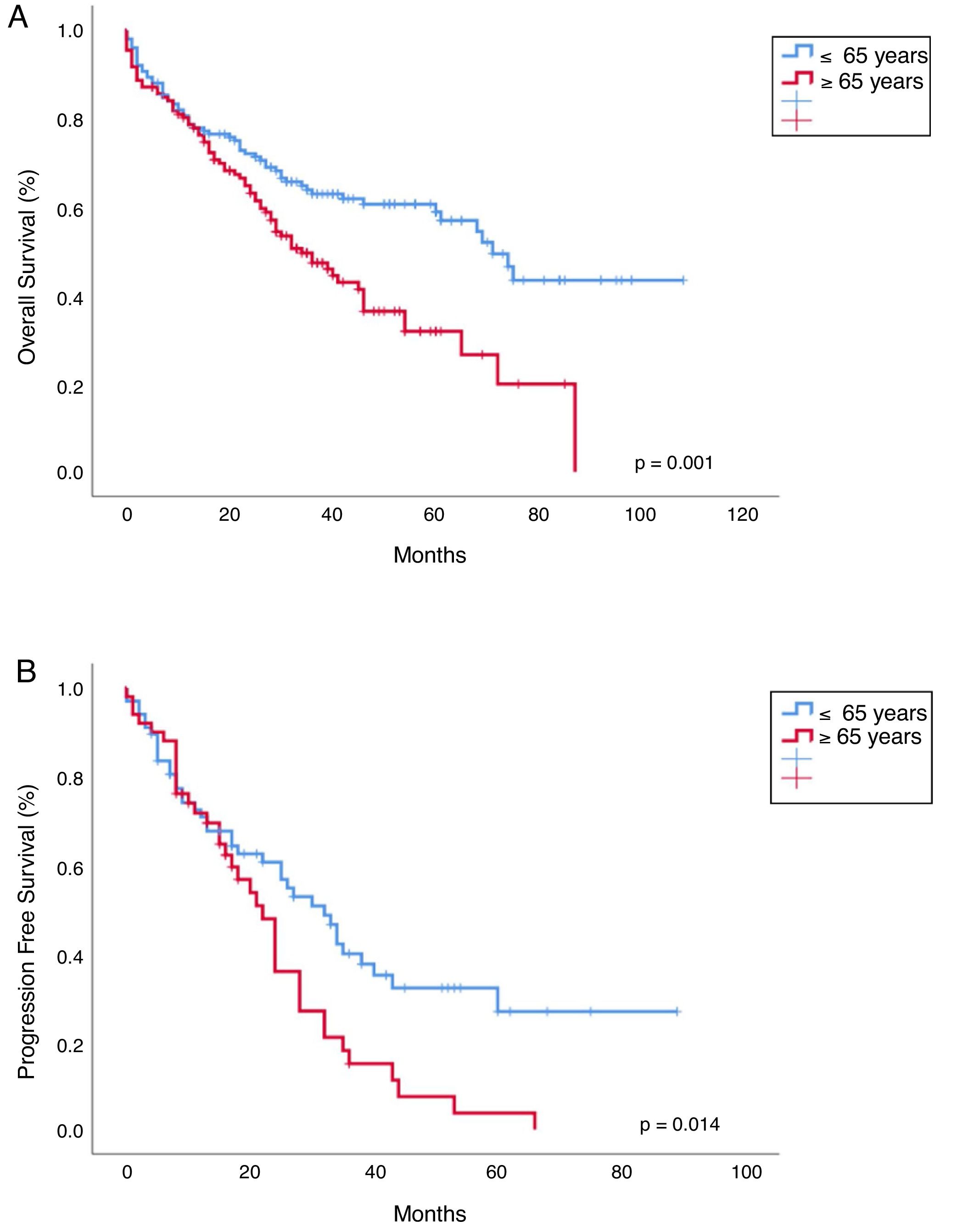
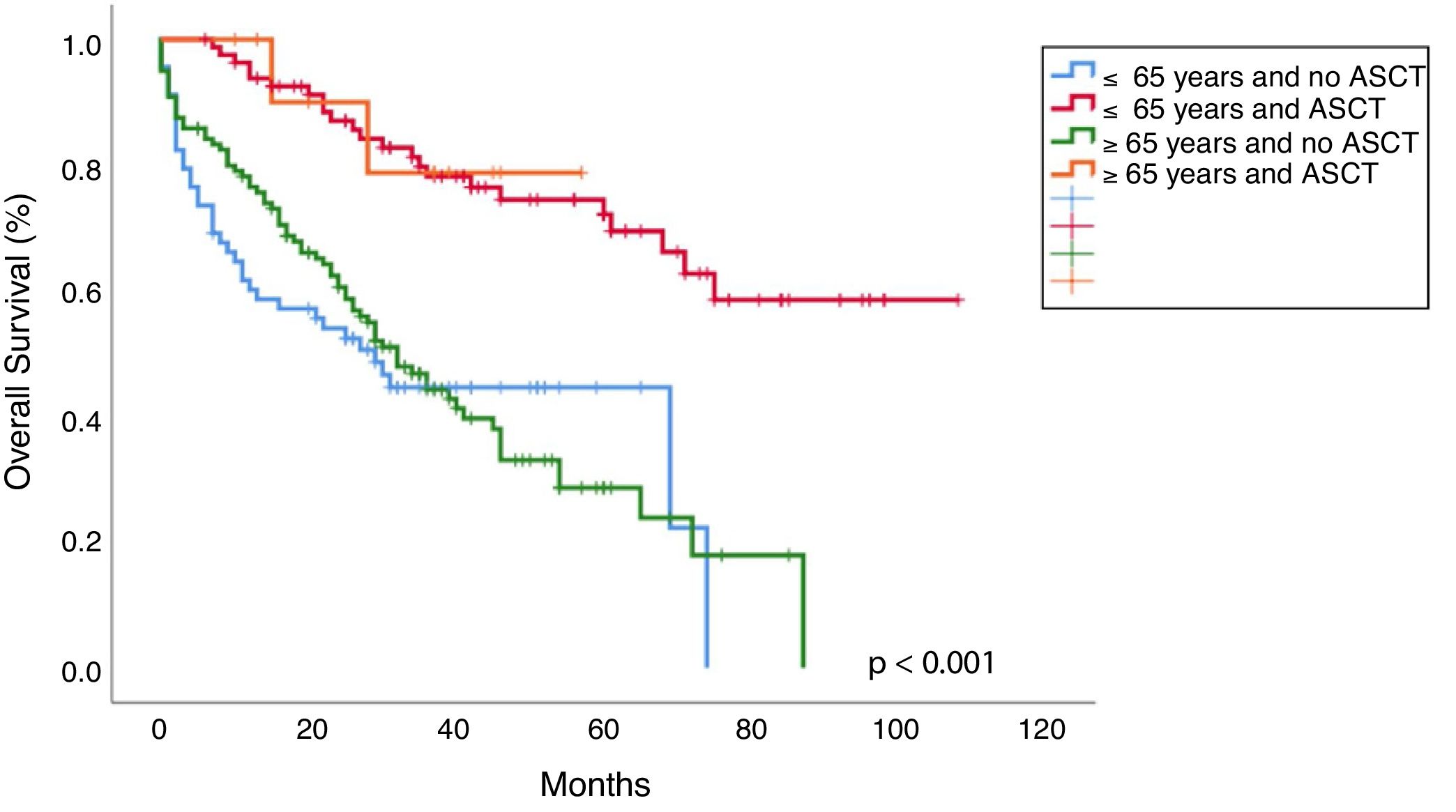
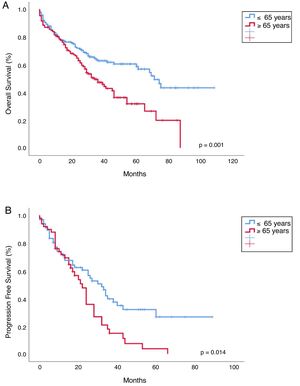
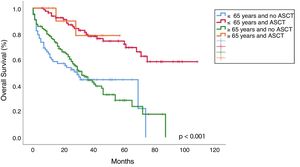
Progression free survival, overall survival and mortality rateWith a median follow-up of 30 months (range 0–108), median overall survival (OS) was 65 months vs 41 months, favoring young patients (p=0.001; Figure 2). Median progression-free survival (PFS) in young patients was 40 months and 23 months in the old (p=0.014; Figure 2). The OS in patients younger than 65 years who received ASCT vs no ASCT were 80 months and 37 months, respectively, and in the older were 50 months and 39 months, respectively (p<0.001; Figure 3).
The mortality rate was 40.7% (61/150) in the young and 57.6% (76/132) in the old (p=0.005). The main cause of death was disease progression in both groups, particularly in the young (60% vs 41.1%; p=0.095). Infections were the second most frequent cause of death in the older patients (16.7% in the young vs 24.7% in the old; p=0.095).
Early mortality (before 6 months) occurred in 12% (18/150) and 14.4% (19/132) of the young and older patients, respectively (p=0.552). Similarly, the main causes were progression and infection in the young and older patients, respectively (progression: 61.1% vs 26.3%; infection: 33.3% vs 57.9%; p=0.096, respectively).
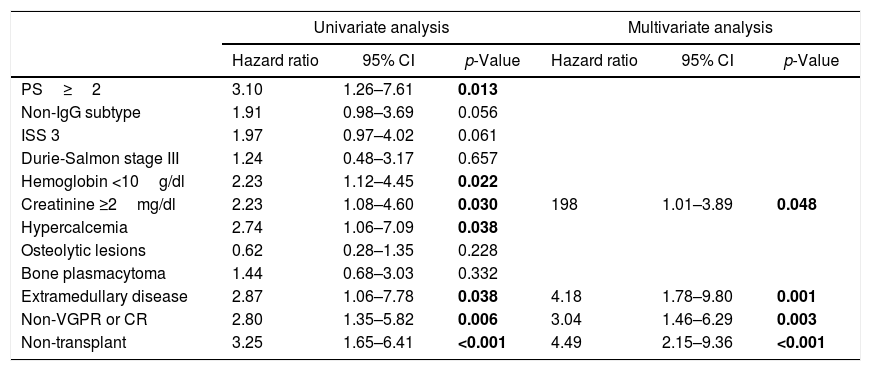
Features significantly associated with survival in the univariate analysis in young patients were PS ≥2 (p=0.013), hemoglobin <10g/dl (p=0.022), creatinine ≥2mg/dl (p=0.030), calcium >11mg/dl (p=0.038), extramedullary disease (p=0.038), non-VGPR or CR (0.006) and non-ASCT (p<0.001) (Table 2). In the multivariate analysis, creatinine ≥2mg/dl (p=0.048), extramedullary disease (p=0.001), non-VGPR o CR (p=0.003) and non-ASCT (p<0.001) were identified as independent prognostic factors for shorter survival (Table 2).
Univariate and multivariate analysis of factors associated with survival in patients 65 years or under.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-Value | Hazard ratio | 95% CI | p-Value | |
| PS≥2 | 3.10 | 1.26–7.61 | 0.013 | |||
| Non-IgG subtype | 1.91 | 0.98–3.69 | 0.056 | |||
| ISS 3 | 1.97 | 0.97–4.02 | 0.061 | |||
| Durie-Salmon stage III | 1.24 | 0.48–3.17 | 0.657 | |||
| Hemoglobin <10g/dl | 2.23 | 1.12–4.45 | 0.022 | |||
| Creatinine ≥2mg/dl | 2.23 | 1.08–4.60 | 0.030 | 198 | 1.01–3.89 | 0.048 |
| Hypercalcemia | 2.74 | 1.06–7.09 | 0.038 | |||
| Osteolytic lesions | 0.62 | 0.28–1.35 | 0.228 | |||
| Bone plasmacytoma | 1.44 | 0.68–3.03 | 0.332 | |||
| Extramedullary disease | 2.87 | 1.06–7.78 | 0.038 | 4.18 | 1.78–9.80 | 0.001 |
| Non-VGPR or CR | 2.80 | 1.35–5.82 | 0.006 | 3.04 | 1.46–6.29 | 0.003 |
| Non-transplant | 3.25 | 1.65–6.41 | <0.001 | 4.49 | 2.15–9.36 | <0.001 |
The values in blod highlight that these variables had a significant difference (p<0.05).
This analysis provides information regarding the impact of age on a real-life cohort of active, NDMM patients in the era of novel treatments in Latin America. Similar to international reports, 53.2% of the patients were ≤65 years, 36% ≤60 years and 15.9% ≤50 years.2,4 Globally, patients present with renal failure, high DS stage and ISS 3, reflecting late diagnosis, regardless of age. A higher percentage of ISS 1 was found in young patients (25% vs 10.9%; p=0.004), probably due to a higher level of albumin. An advanced Durie-Salmon stage was more frequently in young patients, compared with the older (85.3% vs 73.5%; p=0.013). This finding is similar to that reported by other studies. In the International Myeloma Working Group study, patients younger than 50 years had more frequently ISS 1 (39% vs 26%; p<0.001), but also more Durie-Salmon stage III (60% vs 53%; p<0.001) than the older patients. Similarly, in the Intergroupe Francophone du Mylome (IFM) study patients under 60 years presented more ISS 1 (37.2% vs 30%; p<0.007). However, a higher percentage of Durie-Salmon stage III and ISS 3 in the young was found in our analysis, compared to international reports (DS III 85.3% vs 50–68% and ISS 3 48.5% vs 26–27%, respectively).5,6,11–14
In contrast with international publications, our analysis did not show significant differences in anemia, renal failure, mean monoclonal protein spike and bone marrow plasmacytosis. Ludwig et al. reported a significantly higher frequency of anemia, renal impairment and monoclonal spike in patients younger than 51 years.5 Bladé et al. and Cheema et al. found that more than 25% of patients younger than 40 years presented with renal failure, probably associated with a high percentage of light-chain multiple myeloma (LCMM). In our study, renal failure in the young was 28. This was not attributable to a higher rate of LCMM, as this group was less frequent than IgA in the young.7,15 Nevertheless, renal failure in young MM patients was higher than that reported internationally (10–15%).5,13,14 Osteolytic lesions and bone plasmacytomas were significantly more frequent in young patients, which differs from other publications.5,13
Extramedullary disease was more frequently diagnosed in young patients (12.7% vs 0%; p<0.001). Bladé et al. reported 19% of extramedullary plasmacytomas in patients younger than 40 years.7 Imaging techniques used to evaluate patients at diagnosis were not detailed in our study. Differences in the use of X-rays, CT scan, PET-CT and MRI in both groups may explain these findings. In the real-life setting, more in-depth imaging evaluation tends to be made for the young patients and this might explain the aforementioned differences. This clearly merits further research.
Cytogenetic abnormalities were found at a low percentage in both groups, probably related to the lack of availability of plasma cell selection in 2/3 of the institutions included in the analysis. As previously reported, lack of sorting is frequent in Latin America.16 Adequate cytogenetic analysis is necessary to select the most appropriate therapy.
In spite of the higher use of bortezomib-based therapy in young patients, the overall response rate (≥PR) and depth of response (VGPR+CR) were not statistically different between the groups. Despite its availability, the upfront ASCT rate is low (57.7% in young and 46.5% in <71 years). Reasons for 42.3% of young patients not undergoing ASCT have not been specified in the charts. One of them could be the high frequency of renal impairment and the need for dialysis in young patients. In our country, patients in chronic dialysis do not undergo ASCT. In any case, considering this potent therapeutic strategy is widely available in Uruguay, this finding needs further analysis.
In line with previous publications, in our study the young age was significantly associated with a longer OS (median 65 months vs 41 months; p=0.001) and ASCT prolonged survival in both groups, the young (median OS 80 months vs 37 months; p<0.001) and old patients (median OS 50 months vs 39 months; p<0.001), but this benefit was more pronounced in the younger (Figure 3). This difference in OS between the young and old who received ASCT could be explained by the worse PS and more comorbidities in older (PS≥2: 21.7% vs 36.8%; p=0.011). The IMWG study observed better OS in patients younger than 50 years compared to the older, both after conventional therapy (median, 4.5 vs 3.3 years; p<0.001) and ASCT (median, 7.5 vs 5.7 years; p=0.04).5 Similar findings have been observed by the Nordic Myeloma Study Group in patients younger than 60 years compared with those aged 60–64 years after ASCT (66 months vs 50 months; p<0.001).13 More recently, the Swedish Myeloma Registry showed a shorter OS in older patients (>66 years), compared to the younger (3.4 years and 7.7 years, respectively) and the IFM showed a longer OS in those younger than 60 years, compared to patients aged 60–65 (p=0.003).4,6
In contrast, others studies did not reveal significant differences in survival between younger and older patients after ASCT.11,12,15 Cheema et al., did not find significant differences in OS after ASCT between patients 40 years old or under and those 41–65 years old (68.1 months vs 80.7 months, respectively; p=0.9). This study included patients with de novo plasma cell leukemia (PCL), which may contribute to these results.15
Multivariate analysis was performed in young patients, excluding those with one or more missing values in all factors. Creatinine ≥2mg/dl (p=0.048), extramedullary disease (p=0.001),p=0.003) and non-ASCT (p=0.048) were independent risk factors for shorter survival (Table 2). Increased serum creatinine and non-ASCT have been shown to be adverse factors in others series.5 Extramedullary disease has been associated with poor survival.17–19 In contrast with other publications, the ISS score was not shown to have an impact on survival.5,6
The main cause of death was MM progression in both groups. An early mortality rate was not different between the young and old and the main cause of early death in the older were infections. Factors associated with a higher rate of infection in the older will be addressed in future research.
This study has limitations, particularly attributable to its retrospective nature. Lack of detailed information regarding imaging techniques, other causes of renal failure and comorbidities influencing infections and OS are the most relevant deficiencies.
In conclusion, although MM patients younger than 66 years of age have an aggressive presentation with an advanced stage, high rate of renal failure and extramedullary disease, this did not translate into an inferior OS and PFS. Young patients live longer, particularly those undergoing ASCT. Older age is an independent adverse prognostic factor. Adequate risk identification, frontline treatment based on novel drugs and ASCT are the best strategies to improve outcomes, both in young and old patients.
Authors’ contributionsV.B. and E.R. contributed in the conception and design of the study, acquisition, analysis and interpretation of data and drafted the article. D.G. contributed to statistical analysis. All authors read and approved the final manuscript.
Conflicts of interestThe author declares no conflicts of interest.
List of participating institutions:
Hospital de Clínicas “Dr. Manuel Quintela”
Asociación Española Primera en Socorros Mutuos
Sociedad Médica Universal
Hospital Británico
Médica Uruguaya
Servicio Médico Integral
CASMU
Hospital Central de las Fuerzas Armadas
Hospital Maciel
Centro de Asistencia Médica de Lavalleja (CAMDEL)
Sociedad Médico Quirúrgica de Salto (SMQS)
COSEM
Gremial Médica Centro Asistencial (GREMCA)
Instituto Asistencial Colectivo (IAC) – Treinta y Tres
Hospital Policial
Círculo Católico de Obreros
Cooperativa Médica de Tacuarembó (COMTA)
Casa de Galicia
Hospital Evangélico
Instituto Nacional del Cáncer (INCA)
Cooperativa Asistencial Médica del Este de Colonia (CAMEC)
Hospital Departamental de Cerro Largo – ASSE
Hospital de San Carlos
Hospital Saint Bois
Asociación Médica San José (AMSJ)
Corporación Médica de Paysandú (COMEPA)
Cooperativa Médica de Florida (COMEF)
Hospital de Bella Unión – ASSE
Sanatorio San Carlos (AMECOM)
Gremial Médica de Artigas – GREMEDA
Sanatorio Mautone