Infections represent a significant cause of morbidity and mortality in patients with multiple myeloma (MM). In Latin America, data on infectious complications in newly diagnosed MM (NDMM) patients are limited.
MethodsWe conducted a multicenter, prospective cohort study of patients with NDMM in Uruguay between June 2019 and December 2020. Patients with active disease, on active therapy and who provided written informed consent were included. Elegible patients were followed for 6 months from the time of diagnosis and before proceeding to autologous stem cell transplantation or until death, whichever occurred first. Our primary endpoint was the number of infectious events that required hospitalization for ≥ 24 h.
Main resultsOf 124 patients with NDMM, 54 (43.5 %) had infectious complications (74 infectious events), the majority (74.3 %) within the first 3 months from diagnosis. The most common sites of infection were urinary (39.2 %) and respiratory tracts (33.8 %). The microbial agent was identified in 60.8 % of patients with Gram-negative bacteria (71.4 %) as the most common pathogen. Viral and fungal infections were infrequent. In the multivariable analysis, the Eastern Cooperative Oncology Group (ECOG) performance status was ≥ 2 (odds ratio [OR], 2.16; 95 % confidence interval [95 %CI], 1.23 - 3.79; p = 0.008) and creatinine ≥ 2 mg/dl (OR, 2.33; 95 %CI, 1.33 - 4.07; p = 0.003) were independent factors associated with bacterial infections. At 6 months, 14 patients (11.3 %) had died, 50 % related to infectious complications.
ConclusionBacterial infections are a substantial cause of hospital admissions and early death in patients with NDMM. Antibiotic prophylaxis should be considered to reduce infectious complications in patients with MM.
The advances in the management of multiple myeloma (MM) have yielded improved outcomes.1,2 However, infections are an important cause of morbidity and the leading cause of death in MM patients, responsible for approximately 50 % of early MM deaths3. A recent study showed a 7- and 10-fold increased risk for the development of bacterial and viral infections, respectively, in MM patients, compared to matched controls.4 Pneumonia and sepsis are the most common infections, typically caused by Streptococcus pneumoniae, Haemophilus influenzae and other gram-negative bacteria.4–8 An impaired cellular and humoral immunity, coupled with demographic features (i.e., older age, frailty and co-existing comorbid conditions) play a role in the increased susceptibility to infections.9 In the last years, the addition of proteasome inhibitors (PIs); and immunomodulatory drugs (IMiDs) to the induction treatment, has shifted the epidemiology of infections to an increased number of events happening earlier during therapy.10
To date, data on the epidemiology of infectious complications in MM patients in Latin America are scarce. Therefore, we aimed to prospectively study the epidemiology of infections and to investigate risk factors associated with the development of infections in patients with newly diagnosed MM (NDMM) within the first 6 months from diagnosis. The identification of clinical and epidemiological characteristics associated with infections may help define the appropriate prophylactic approach to reduce this complication.
MethodsPatientsWe conducted a multicenter, prospective cohort study of all consecutive NDMM patients diagnosed at 4 specialized hematology centers in Montevideo, Uruguay (Hospital Central de las Fuerzas Armadas and Hospital de Clínicas, both public healthcare institutions, Hospital Británico and CASMU-IAMPP, both private healthcare institutions). The inclusion criteria included active disease, being currently on therapy and having provided written informed consent. Patients with monoclonal gammopathy of undetermined significance, smoldering MM, plasma cell leukemia, amyloidosis and HIV infection were excluded. Eligible patients were consecutively enrolled between June 2019 and December 2020, by their own treating physician at each participating center. Thereafter, patients were clinically observed for development of infectious events that required hospitalization for ≥ 24 h for an additional 6 months and before proceeding to autologous stem cell transplantation or until death, whichever occurred first. Demographics, comorbidities, laboratory data and myeloma-specific features were collected on a standardized form. Institutional Review Boards approved this study at each participating institution.
Study variablesData on all infectious events that required hospitalization for ≥ 24 h were recorded. The variables analyzed were the infection site, type of isolated microbial agent, infection severity, time to occurrence, and outcome from the infection. We found 7 cases of SARS-CoV-2 infection, which were excluded from the analysis. Catheter-related infections, but no port-a-cath exit site infections, were included in the analysis. The antimyeloma treatments were defined as IMiD-based (i.e., thalidomide or lenalidomide), PI-based (i.e., bortezomib) and IMiD plus PI-based. The choice of therapy and antimicrobial prophylaxis were decided by the treating physician. Comorbidities included in the analysis were diabetes mellitus, chronic pulmonary disease, asthma and heart failure.
DefinitionsThe diagnosis of MM was defined according to the International Myeloma Working Group (IMWG) 2014 criteria and staging was performed in adherence to the International Staging System (ISS) recommendations. We defined the infectious event as the presence of a body temperature ≥ 38 ºC, and/or the presence of clinical symptoms or signs of infection. Events were classified as clinically defined (CD) when there was clinical evidence, but microbial isolation was negative; microbiologically defined (MD), when the microbial agent was identified from a blood test and/or other body sources; and fever of unknown origin, when the only clinical sign was fever without microbial isolation. The type of infection (i.e., bacterial, viral or fungal) was defined based on combined clinical, imaging and microbiological findings. Bacterial infections were identified by conventional culture methods and enzyme immunoassay in stools was used to identify the Clostridium difficile infection. Culture-independent methods to identify viral and fungal infections (e.g., respiratory viral panel, serum galactomannan, urine histoplasma antigen) were recorded when available. When the infectious agent was not identified, if the response to empiric antibiotic, antifungal or antiviral therapy was documented, they were classified as bacterial, fungal or viral infection, respectively. Early death was defined as death within the first 6 months from diagnosis. The cause of death (classified as either infectious or non-infectious) was determined by the treating physicians.
Statistical analysesDemographics, clinical features and therapies received were summarized using descriptive statistics. The primary study outcome was the number of infectious events that required hospitalization for ≥ 24 h within the first 6 months of the follow-up. Secondary outcomes were the mortality rate at 6 months and its cause. Quantitative variables were described in terms of the median; qualitative variables were described as the absolute percentage. Patients were divided based on the presence or absence of infectious events. Comparisons between subgroups were analyzed using the Chi-square test. Univariate analysis was performed using the Chi-square test to identify possible risk factors for infection; those with a p < 0.05 were selected and included in the multivariate analysis, which was performed using a binary logistic regression model (forward LR). The degree of collinearity between variables was evaluated using the Variance Inflation Factor (VIF) statistic. Clinical and treatment factors evaluated were: age, Eastern Cooperative Oncology Group (ECOG) performance status, smoking habit, comorbidities, myeloma subtype, ISS score, Durie-Salmon stage, anemia (hemoglobin level < 10 g/dl), renal impairment (serum creatinine level ≥ 2 mg/dl), hypercalcemia (serum calcium > 11 mg/dl), presence of osteolytic lesions (one or more on skeletal imaging), lymphopenia (blood lymphocyte count ≤ 1 × 109/L), hypoalbuminemia (serum albumin < 3.5 g/dl), elevated serum lactate dehydrogenase (LDH) (above the upper limit of normal), immunoparesis (decreased serum concentration of any polyclonal immunoglobulin class in serum) and type of therapy. In all cases, p < 0.05 was considered significant. The statistical analysis was performed using the IBM SPSS version 25.0 (Armonk, NY, USA).
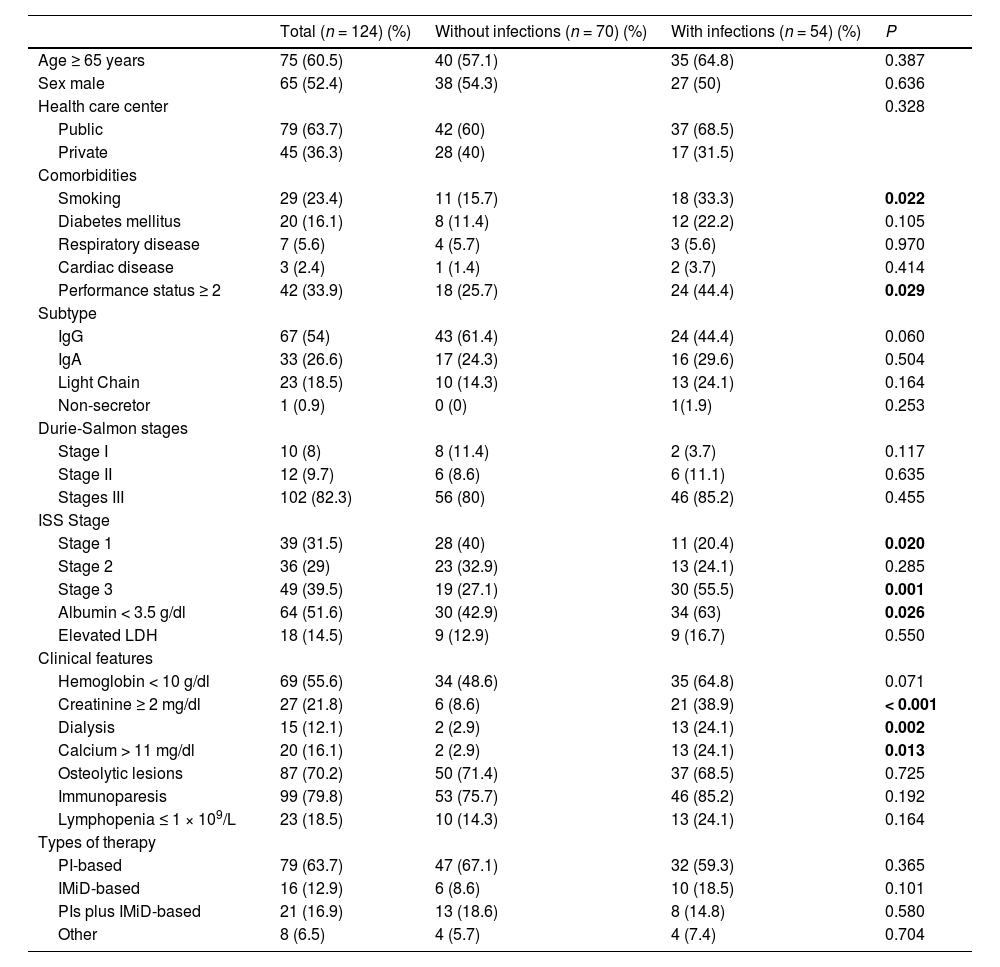
ResultsEpidemiological and clinical featuresA total of 124 patients with NDMM were included (65 males and 59 females). Forty-two (33.9 %) patients were diagnosed at the Hospital de Clínicas, 39 (31.5 %) at the CASMU, 37 (29.8 %) at the Hospital Central de las FF.AA. and 6 (4.8 %) at the Hospital Británico. The median age at diagnosis was 68 years (range, 27 - 88), 75 (60.5 %) patients were ≥ 65 years. One-third of the patients had an ECOG performance status ≥ 2. A total of 67 patients (54 %) were IgG subtype, 33 (26.6 %) IgA, 23 (18.5 %) light chains and 1 non-secretor. Most patients were diagnosed with advanced Durie-Salmon stage III (n = 102, 82.3 %), only 12 patients (9.7 %) were stage II and 10 were stage I (8 %). According to the ISS, 39 were stage 1 (31.5 %), 36 were stage 2 (29 %) and 49 were stage 3 (39.5 %). Bone disease was the most frequent myeloma-defining event (n = 87, 70.2 %), followed by anemia (n = 69, 55.7 %), renal failure (n = 27, 21.8 %) and hypercalcemia (n = 20, 16.1 %). The clinical features of NDMM patients are shown in Table 1.
Characteristics of patients at diagnosis.
Abbreviations: ISS, International Staging System; LDH, lactate dehydrogenase; Pis, proteosome inhibitors (i.e., bortezomib); IMiDs, immunomodulatory drugs (i.e. thalidomide and lenalidomide).
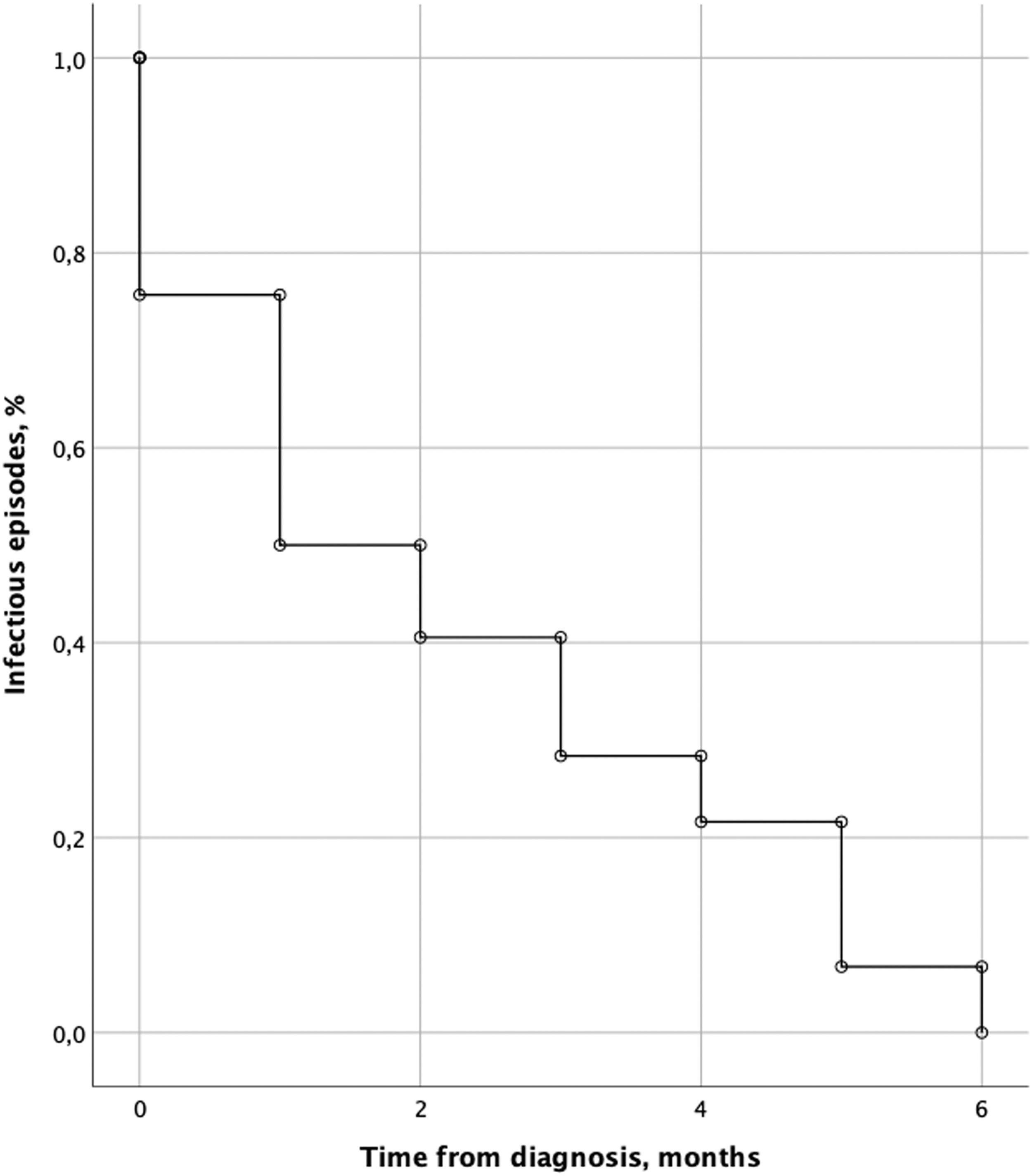
Infections were found in 54 patients (43.5 %) with a median time to the first infection of 1 month from diagnosis (range 1 - 6 months). A total of 74 infectious events were identified in the 54 patients; 31.5 % (n = 17) has ≥ 2 infectious events. The majority of infectious events (n = 55/74, 74.3 %) occurred in the first 3 months from diagnosis, particularly within the first month (n = 37/74, 50 %). The distribution of infectious episodes over time from the diagnosis is shown in Figure 1.
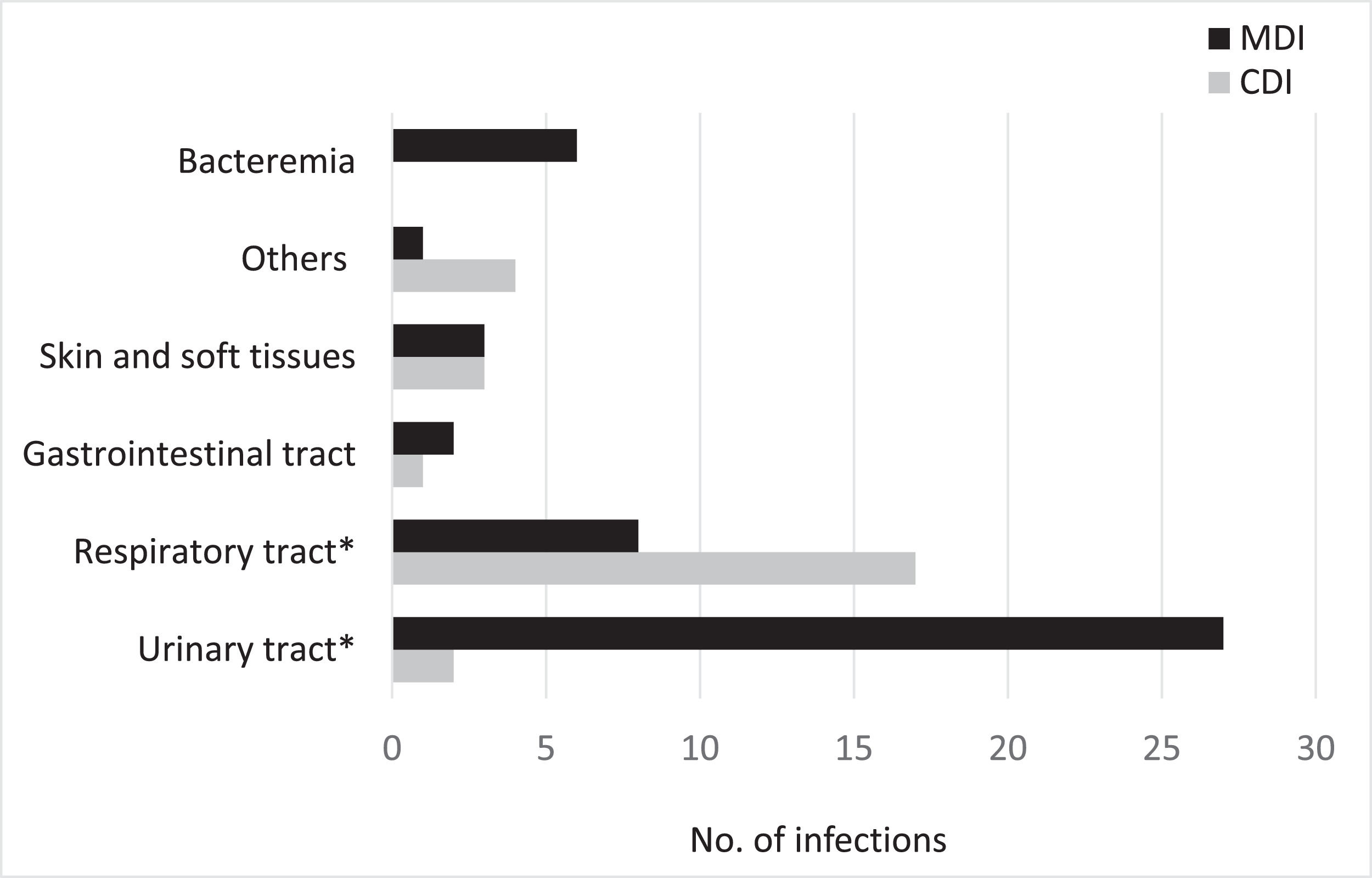
The most common site of infection was the urinary tract (n = 29, 39.2 %), followed by the respiratory tract (n = 25 cases, 33.8 %), skin and soft tissue (n = 6, 8.1 %), blood stream (n = 6, 8.1 %), gastrointestinal tract (n = 3, 4.1 %) and central nervous system (n = 2, 2.7 %). In 3 cases (4.1 %), the site of infection was not identified and they were classified as a fever of unknown origin. Respiratory infections were predominantly CD (p < 0.001), whereas urinary tract infections were MD (p < 0.001). The distribution of infected sites is summarized in Figure 2.
Overall, 83.1 % of patients received antiviral prophylaxis (100 % of those treated with bortezomib). Prophylaxis with fluoroquinolones, trimethoprim-sulfamethoxazole and fluconazole were used in 6.5 %, 2.4 % and 0.8 %, respectively. The immunization against H. influenza and S. pneumoniae was documented in 50.8 % (n = 63) and 27.4 % (n = 34) of patients, respectively.
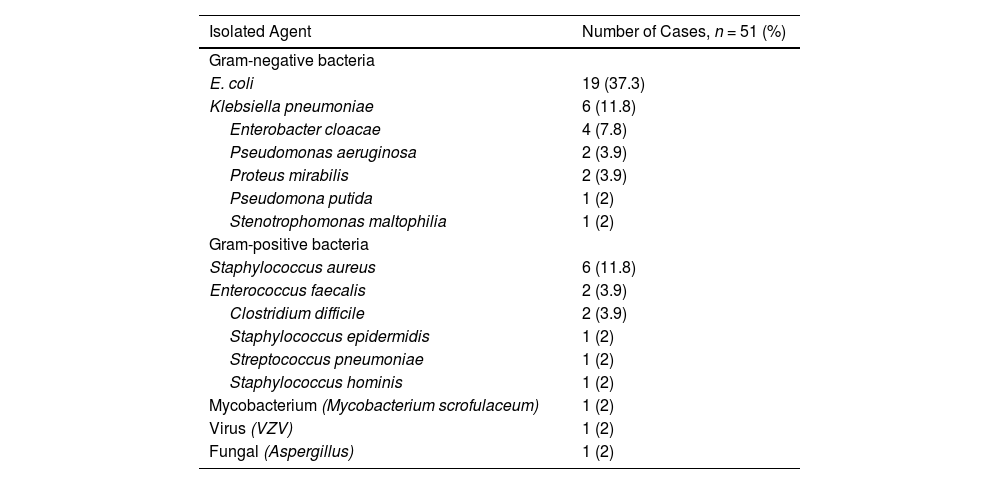
Distribution of the pathogensIn the 74 infectious events, the microbial agent was isolated in 45 (60.8 %) cases; 6 (13.3 %) had more than one microorganism isolated. Bacterial infections represented 94.6 % of the episodes. Viral and fungal infections were infrequent. Gram-negative bacteria represented 71.4 % (n = 35/49) and Gram-positive bacteria, 26.5 % (n = 13/49) of the MD cases. One case corresponded to mycobacterium infection (2 %, 1/49). The most frequent pathogen was Escherichia coli (37.3 %), followed by Klebsiella pneumoniae (11.8 %) and Staphylococcus aureus (11.8 %) (Table 2). The major sources for microorganism isolation were urine (59.1 %), blood culture (18.2 %) and bronchoalveolar lavage (9.1 %).
The frequencies of isolated agents.
Abbreviations: VZV, Varicella zoster virus.
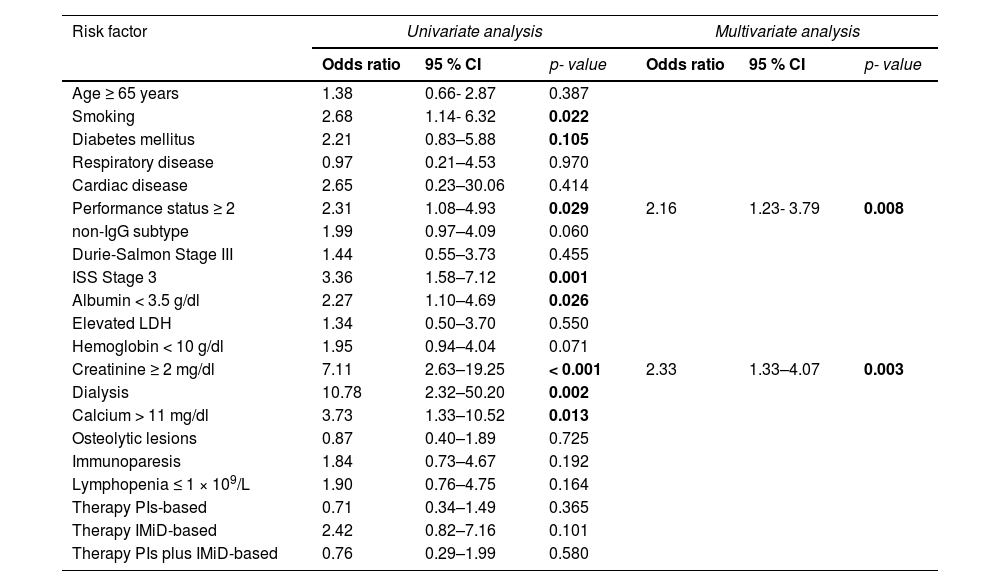
According to the univariate analysis, the factors associated with a higher risk for bacterial infection in the first 6 months from diagnosis were: ECOG performance status ≥ 2 (p = 0.029); smoking habit (p = 0.022); ISS 3 (p = 0.002); creatinine ≥ 2 mg/dl (p < 0.001); dialysis (p = 0.002); hypercalcemia (p = 0.013) and; serum albumin < 3.5 g/dl (p = 0.026) (Table 3). An age ≥ 65 years, the gender, presence of co-existing comorbidities, such as diabetes, respiratory and cardiac diseases, Durie-Salmon stage III, non-IgG MM subtype, presence of anemia, osteolytic lesions, immunoparesis and lymphopenia did not show significant differences between those who developed infections versus those who did not (Table 1).
Univariate and multivariate analysis of risk factors associated with bacterial infections in patients with newly diagnosed multiple myeloma.
Abbreviations: ISS, International Staging System; LDH, lactate dehydrogenase; PIs, proteosome inhibitors (i.e., bortezomib); IMiDs, immunomodulatory drugs (i.e., thalidomide and lenalidomide).
To analyze the effect of the MM therapy on the rate of infection, we categorized cases according to the drug for which the regimen was based (i.e., PI-based, IMiD-based and the combination of both). The PI-based therapy was administered in 79 cases (63.7 %), IMiD-based therapy in 16 cases (12.9 %) and a combination of both in 21 patients (16.9 %). The remaining cases were treated with conventional chemotherapy (Table 1). According to therapy type, we did not find a significant difference in the risk of infection (Table 3).
In the multivariate logistic regression analysis (Table 3), the factors with an independent prognostic value for the development of bacterial infections were an ECOG performance status ≥ 2 (0.008) and creatinine ≥ 2 mg/dl (p = 0.003). There was no collinearity among the factors (VIF < 2 in all cases).
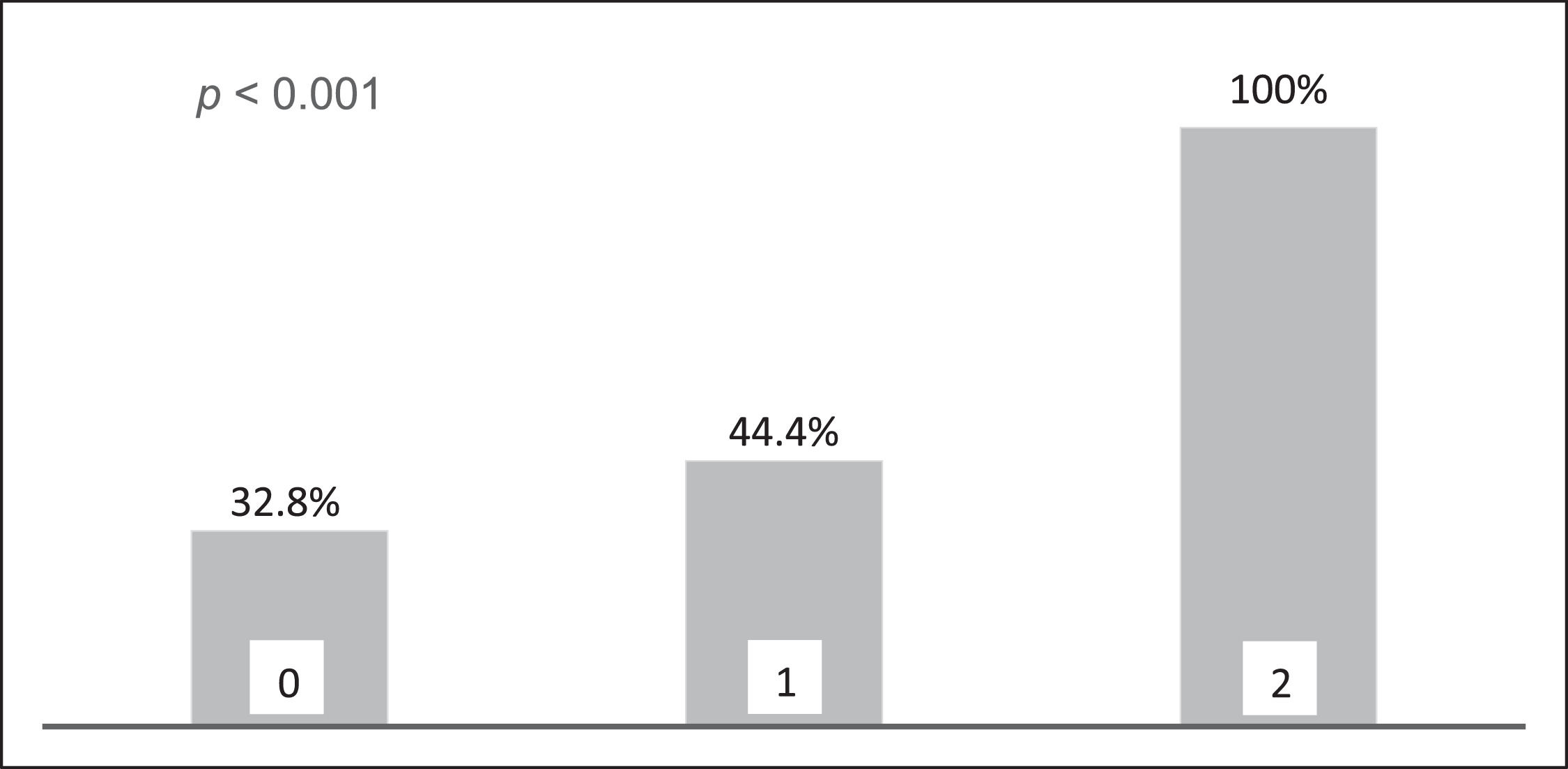
The rate of infection was 32.8 % in the absence of risk factors, 44.4 % with 1 risk factor, and 100 % with 2 risk factors (p < 0.001, Figure 3). Analysis of risk factors associated with fungal and viral infections was not performed, given the small sample size.
Intensive care unit admission and mortality rateOverall, 18.9 % (n = 14/74) of infectious events resulted in admission to the intensive care unit. A total of 14 (11.3 %) patients died within 6 months of their diagnosis; 7 (50 %) of these deaths were due to infectious complications.
DiscussionAround 44 % of NDMM patients experienced infectious complications early on in their treatment, particularly in the first 3 months from diagnosis. With a median follow-up of 6 months, the overall mortality rate was 11.3 %, half of these deaths being due to infections. This study confirms infections as a major cause of morbidity and early mortality in this patient population and highlights the importance of preventing infectious complications early during MM management.
Patients with MM experience a higher rate of infection, compared to the general population,3,4 particularly in the first two months of induction therapy.11–14 This may be explained by the immunosuppressive nature of active disease added to the immunosuppressive effect of antimyeloma agents.3,15 In our study, the majority of infectious complications were bacterial and caused by Gram-negative bacteria, which is concordant with previously published data. However, contrary to existing reports that showed a higher incidence of respiratory infections, the most frequent site of infection was the urinary tract.5,14,16,17 This finding could be explained by the SARS-CoV-2 pandemic (during this period, respiratory care measures were more rigorous). SARS-CoV-2 infectious events were excluded from the analysis because when we started enrolling patients, there was no SARS-CoV-2 in our region and due to the heterogeneity in the diagnosis and management at the time of the recruitment, we decided not to include them. Historically, a high risk of infection with encapsulated bacteria has been reported in MM patients. In recent studies, the frequency of infections due to S. pneumoniae and H. influenzae has been low, representing only 5 to 9 % and 2 %, respectively.5,8,16,17 In line with these results, our study found S. pneumoniae in 2 % of all isolations, suggesting that in patients treated in the era of PIs and IMIDs, infection with encapsulated bacteria is relatively low, even in a population in which pneumococcal vaccination is not routinely performed. Although response to immunizations is frequently impaired in patients with MM, pneumococcal vaccines are effective in reducing the risk of pneumonia, therefore routine vaccination against S. pneumoniae and H. influenzae is recommended.18–21
Blimark et al. found that viral infections were ten-times higher in MM patients, compared to matched controls.4 The APEX study described an increased incidence of varicella zoster virus (VZV) reactivation in bortezomib-treated patients.22 In our study, viral infections were infrequent, with only one case of VZV reactivation; a high adherence to antiviral prophylaxis in PI-treated patients may explain the low incidence of VZV reactivation in our cohort. Studies have reported a low incidence of fungal infections in MM patients, with invasive fungal disease documented in less than 2.4 % of the cases, mostly during disease progression.23 Consistent with this, our study found only one case of fungal infection after 6 months of follow-up.
Data on the risk for infection with the use of IMiDs and PIs are conflicting.4,5,24 Recently, Lim et al. reported use of PI-based therapy and increasing lines of therapy were independently associated with an increased risk of infection. However, IMiD-based therapy was not associated with an increased risk.25 Meanwhile, a study conducted by the Grupo de Estudio Latinoamericano de Mieloma Múltiple (GELAMM) found that the use of IMiDs was associated with an increased risk for infections NDMM (OR 3.56, p = 0.003), but a lower use of antimicrobial prophylaxis in patients who received IMiDs might explain the observed outcome.26 Some authors recommended antibacterial prophylaxis, particularly in patients receiving IMiDs.18 In line with Brioli et al., our study found that the use of IMiDs and PIs were not associated with a significantly increased risk of infection.24
Some prospective studies have evaluated the role of prophylactic antimicrobials in MM patients. A randomized study on 212 NDMM patients evaluated prophylactic antibiotics during the first 2 months of treatment, and found no significant differences on the incidence of severe bacterial infections in patients receiving ciprofloxacin, trimethoprim-sulfamethoxazole or in those only under observation.27 A phase III study in 977 NDMM patients, however, showed levofloxacin was associated with a significant reduction of febrile episodes and deaths, compared to the placebo. Based on these results, Drayson et al. suggested that levofloxacin prophylaxis could be used in NDMM during the first 12 weeks of anti-myeloma therapy.28 In our cohort, antimicrobial prophylaxis was used in a low number of patients, therefore we do not have conclusions in this regard.
A large number of studies have shown that the advanced ISS stage is an important risk factor for infection in MM patients.5,8,29,30 In agreement with previous reports, patients in our study who developed infections had a significantly more advanced ISS stage. Smoking and hypoalbuminemia were also more frequent in patients developing infections. Poor performance status and renal impairment have been reported as poor prognostic factors for survival, which increases treatment-related toxicity and the risk of infection in NDMM.3,31,32 Huang et al., showed that the ISS stage 3 and ECOG > 2 were independent risk factors for blood stream infection (BSI) in patients with NDMM and more severe anemia (Hb < 10 g/dl) and worse renal function were influencing factors associated with BSI.7 In the multivariable analysis, our study found that the ECOG performance status of ≥ 2 (p = 0.008) and renal impairment (p < 0.001) were independently associated with an increased risk for bacterial infections. These findings support that tumor burden, disease severity and poor medical condition could potentially explain the increased susceptibility to infections in NDMM.5,7,8,33
Unlike other studies, immunoparesis, elevated LDH and lymphopenia were not significantly associated with an increased risk of infection.8,13,33,34 Although immunoparesis seemed the most logical risk for infection, a study in NDMM showed that infection does not appear to be the main mechanism through which immunoparesis affects survival in NDMM patients.35
Based on all the above, we suggest antibacterial prophylaxis in NDMM cases that have one or both of the risk factors described above (ECOG performance status of ≥ 2 and creatinine ≥ 2 mg/dl) and during at least the first 6 months of induction therapy. A similar recommendation has recently been suggested by the IMWG, who have recommended antibacterial prophylaxis in patients with high tumor burden (ISS 2 - 3), high concentrations of serum LDH, poor performance status and impaired renal function.19
Our study has limitations. First, the voluntary nature of recruiting participating centers may have unintentionally biased patient selection. Moreover, the inclusion of only infections leading to hospitalization, the absence of a centralized laboratory review, the lack of standardized workflow protocols in patients with suspected infections, as well as the heterogeneity of methods used for microbiological characterization, could have led to the underestimation of the frequency of infectious events, the causative microorganism and/or an incomplete characterization of the spectrum of infections in our study population. In addition, the heterogeneity in the management of infectious episodes may have influenced the outcomes. Nonetheless, the main strengths of this analysis are its prospective nature and the inclusion of patients treated only at specialized tertiary centers. Moreover, the outcomes of this study are consistent with those reported internationally. To our knowledge, this is the first study investigating the spectrum of infections in patients with NDMM in our region. We believe that the identification of patients who have a higher risk for developing infections could improve outcomes in myeloma patients.
ConclusionIn conclusion, this study shows that bacterial infections are a substantial cause of morbidity and early mortality in NDMM patients. A rationale for choosing the optimal infection prevention strategy is highly needed, considering the emergence of antimicrobial-resistant strains due to the indiscriminate use of antibiotics. This document raises a concern regarding the impact of early infectious complications in NDMM in our region.