This study evaluated outcomes and risk factors for COVID-19 in 91 Brazilian multiple myeloma (MM) patients between April 2020 and January 2022.
ResultsOf the 91 MM patients diagnosed with COVID-19, 64% had comorbidities and 66% required hospitalization due to COVID-19, with 44% needing ventilatory support and 37% intensive care. Age (OR 2.02; 95%CI 1.02 – 7.7) and hypertension OR 4.5; 95%CI 1.3 – 15.5) were independently associated with hospitalization and certain MM therapies (corticosteroids and monoclonal drugs) were associated with ventilatory support (OR 4.3; 95%CI 1.3 – 14 and OR 5.7; 95%CI 1.8 – 18, respectively), while corticosteroids and immunomodulatory drugs were linked to ICU admission (OR 5.1; 95% CI 1.4 – 18 and OR 3.4; 95%CI 1.1 – 10, respectively). The overall mortality rate was 30%, with the highest rate observed in the ICU (73%). Additionally, the ECOG performance status was linked to increased mortality (OR 11.5; 95%CI 1.9 – 69). The MM treatment was delayed in 63% of patients who recovered from COVID-19.
ConclusionsThe findings highlight the need for preventing COVID-19 and prioritizing vaccination among MM patients, as they have high rates of severe outcomes in the event of COVID-19. It is also essential to monitor the potential clinical impacts of COVID-19 on MM patients in the long-term. Given the limited resources available in treating MM patients in Brazil during the COVID-19 pandemic, outcomes might be worse in this population.
Multiple myeloma is characterized by severe immunosuppression, leaving patients at high risk of infections and of developing severe and life-threatening complications. Infection risk often relates to disease-related immunoparesis, age, comorbidities and systemic therapy.1-3 The COVID-19 pandemic has impacted oncological care, including delays in diagnosis and treatments, but, additionally, regarding several cases of COVID-19 infections in this high-risk population.4 For patients in treatment for multiple myeloma, the same was observed.5 The MM patients showed a higher risk for the SARS-CoV-2 infection and a higher excess mortality in 2020 (difference in excess mortality) than non-MM patients.6 The outcome can be even worse in Brazil, where the scenario is of restricted resources to treat MM patients and large numbers of COVID-19 cases and related deaths.
In this cooperative study, we aimed to assess risk factors and outcomes of COVID-19 in Brazilian patients with MM and describe the impact of the infection on the MM treatment.
MethodsThis was a cooperative retrospective multicenter study performed nationally by the “Grupo Brasileiro de Mieloma” (GBRAM). Patients with multiple myeloma followed at the participating centers and diagnosed with COVID-19 (SARS-CoV 2 polymerase chain reaction (PCR)- positive test) from April 2020 to Jan. 2022 were included in this study. The study included cases managed in and out of the hospital reported by 12 centers in 8 different states, representing 4 of the 5 Brazilian regions.
This research was conducted following the Declaration of Helsinki and was approved by the Ethical Committee of each center under CAAE number 30907420.1.0000.5455.
The medical records of the patients were used to collect the following baseline characteristics: time from diagnosis to COVID-19 infection, age at COVID-19 event, ECOG performance status, comorbidities and myeloma International Staging System (ISS) at MM diagnosis. The current MM therapy and previous stem cell transplant data were also collected. 44
Regarding COVID infection, we collect data concerning symptoms of infection onset and COVID-19 vaccination status.
Clinical features and risk factors were analyzed with the severity of COVID-19 and the following outcomes: hospital admissions, intensive care unit (ICU) admission, ventilatory support, and death. MM treatment modification due to COVID-19 was also accessed.
Statistical analysesCategorical and continuous numeric variables were explored using the chi-square or Fisher exact test, Wilcoxon rank-sum test, or Spearman correlation test; the results were expressed as frequencies and medians. Crude and adjusted odds ratios (OR) were estimated with logistic regression analysis for the outcomes. A p-value lower than 5% was considered significant. All analyses were performed with the IBM SPSS statistical package (SPSS Statistics for MAC, Version 27, IBM Corp).
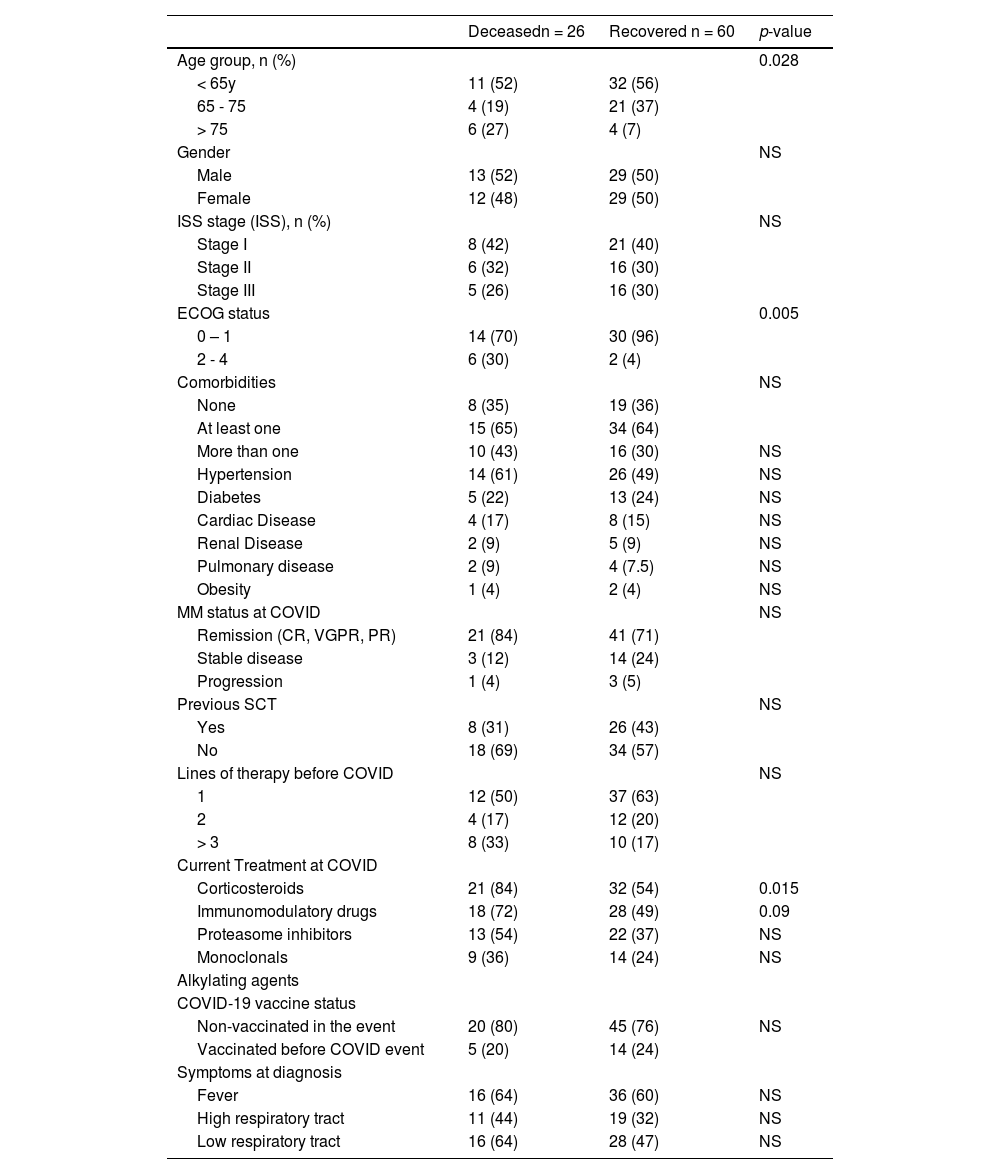
ResultsThe characteristics of the 91 MM patients were reported in Table 1. There was 50% male with a median age of 62 (33 – 84) years and 30% ISS III at diagnosis. At least one comorbidity was present in 52 (64%) patients: most frequently hypertension and diabetes (52% and 23%). Twenty-eight (35%) patients had more than one comorbidity.
Baseline patients characteristics.
Note: percentages of valid cases.
During the COVID episode, 23 (26%) patients had an active or progressing disease, and 37% received at least two prior lines of treatment.
Corticosteroids were the class of MM therapy most frequent used (n=57, 63%), followed by immunomodulatory drugs (n=51, 55%) at the onset of COVID.
COVID-19 infections were classified as at least moderate in (n=60) 66%, and the event management required hospitalization in 60 (66%), ventilatory support in 40 (44%), and ICU admission in 34 (37%). As the cohort started in 2021, most events (74%) occurred in patients not exposed to COVID-19 vaccines.
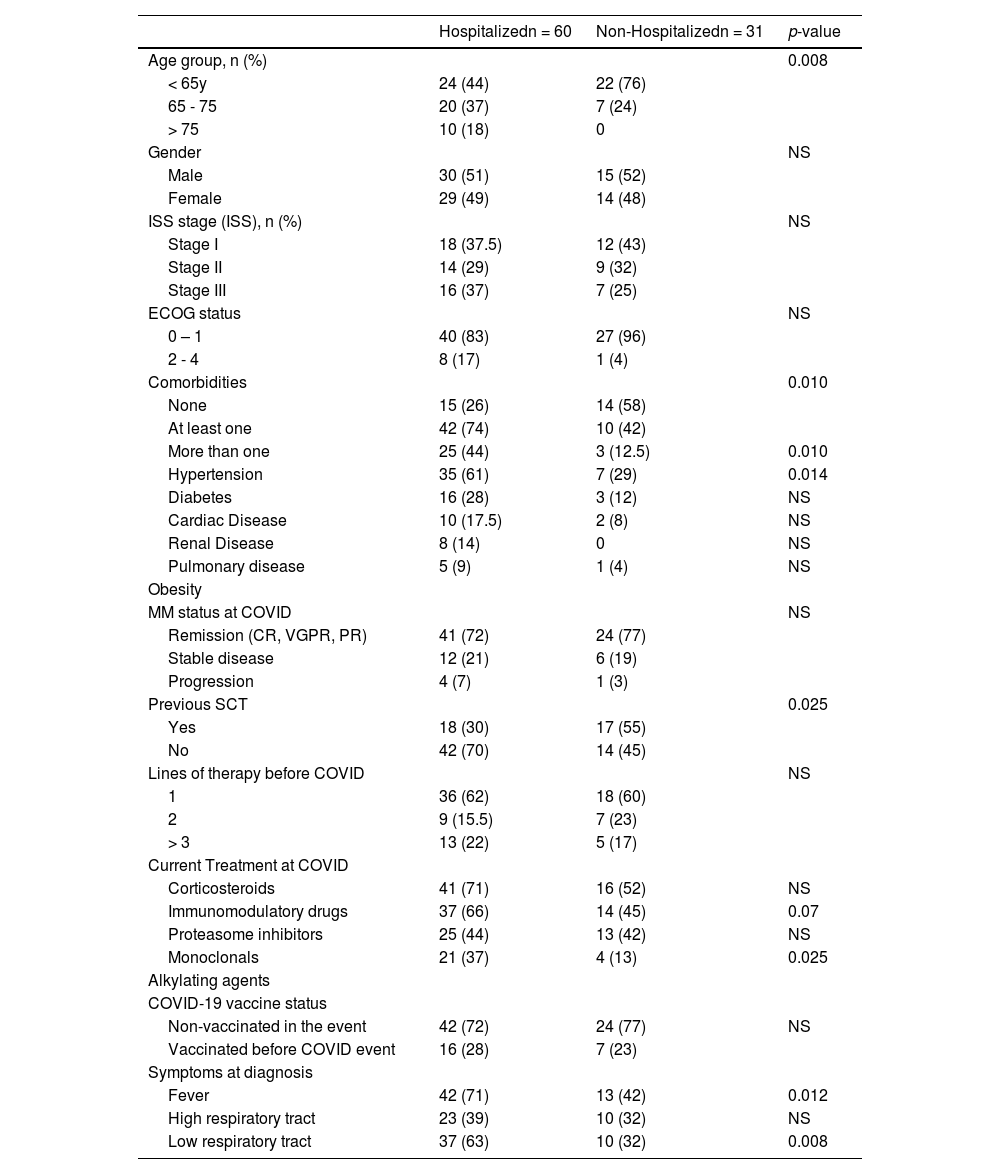
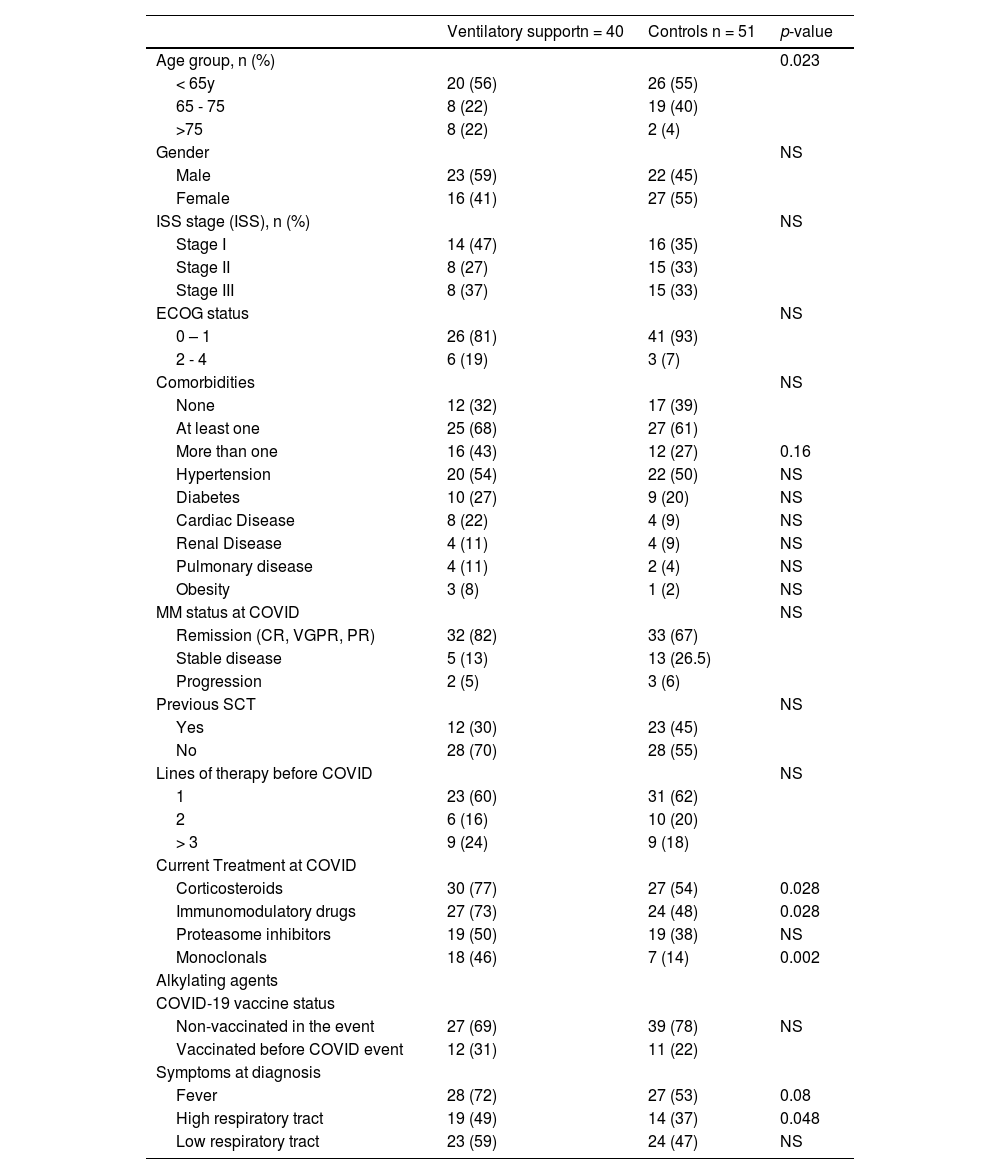
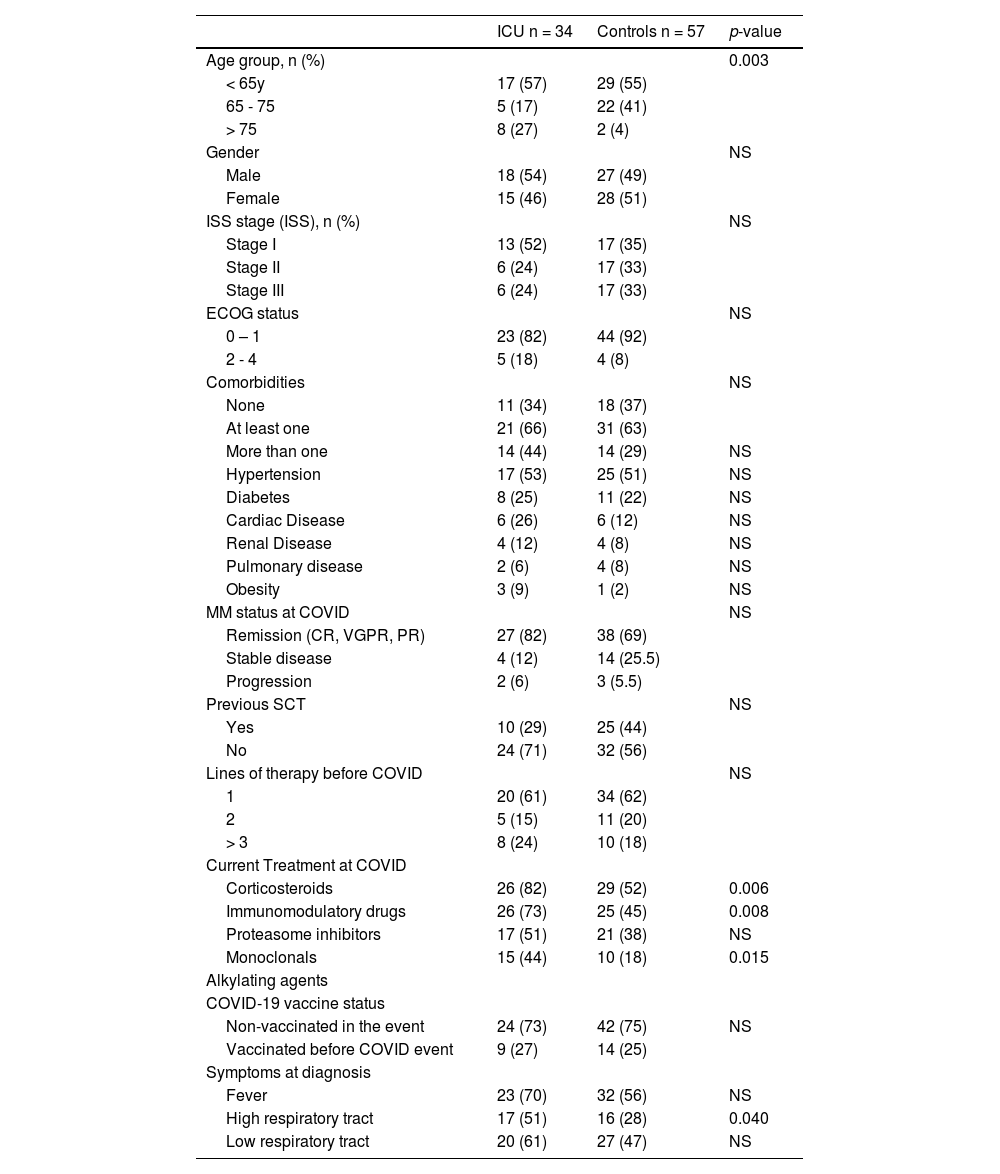
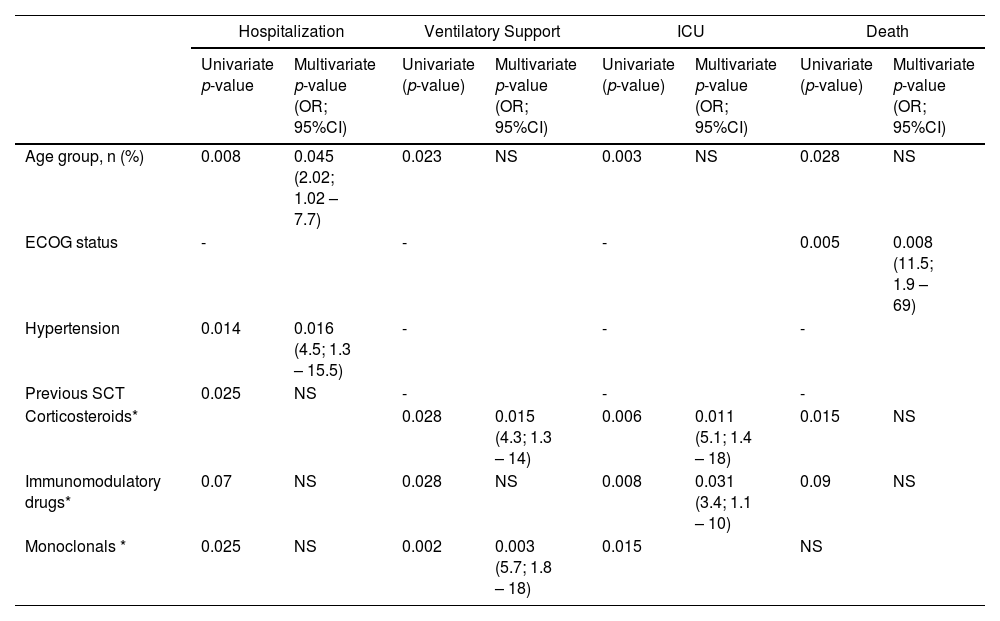
Patients who required hospitalization were older and had more frequent comorbidities. (Table 2) Previous stem cell transplants were less frequent in patients who required hospitalization (30 vs. 55%; p=0.025) compared with controls. The frequency of treatment with immunomodulatory and monoclonals was more frequent in patients who required hospitalization (66 vs. 45%, p=0.07; and 37% vs. 13%, p=0.025) respectively. Ventilatory support was more frequent in older patients (> 75 years) (p=0.02); receiving corticosteroids (p=0.03), immunomodulatory (p=0.03), or monoclonal drugs (p=0.002). (Table 3) Admission in ICU was associated with age (p=0.003), MM treatment including corticosteroids (p=0.006), immunomodulatory (p=0.008), or monoclonal drugs (p=0.015). (Table 4)
Characteristics of patients who required or not hospitalization for COVID care.
Note: percentages and statistics including only valid cases; NS: not statistically significant.
Characteristics of patients who required or not (Controls) ventilatory support for COVID care.
Note: Percentages and statistics including only valid cases; NS: not statistically significant.
Characteristics of patients who required (ICU) or not (Controls) Intensive Care for COVID care.
Note: Percentages and statistics including only valid cases; NS: not statistically significant.
By adjusted multivariate analysis, age and hypertension (p=0.05 and p=0.02), respectively, were independently associated with hospitalization; MM therapy, including corticosteroids and monoclonal drugs (p=0.02 and p<0.01), was related to ventilatory support; treatment with corticosteroids and immunomodulatory drugs (p=0.01 and p=0.03) were associated with ICU admission. (Table 6)
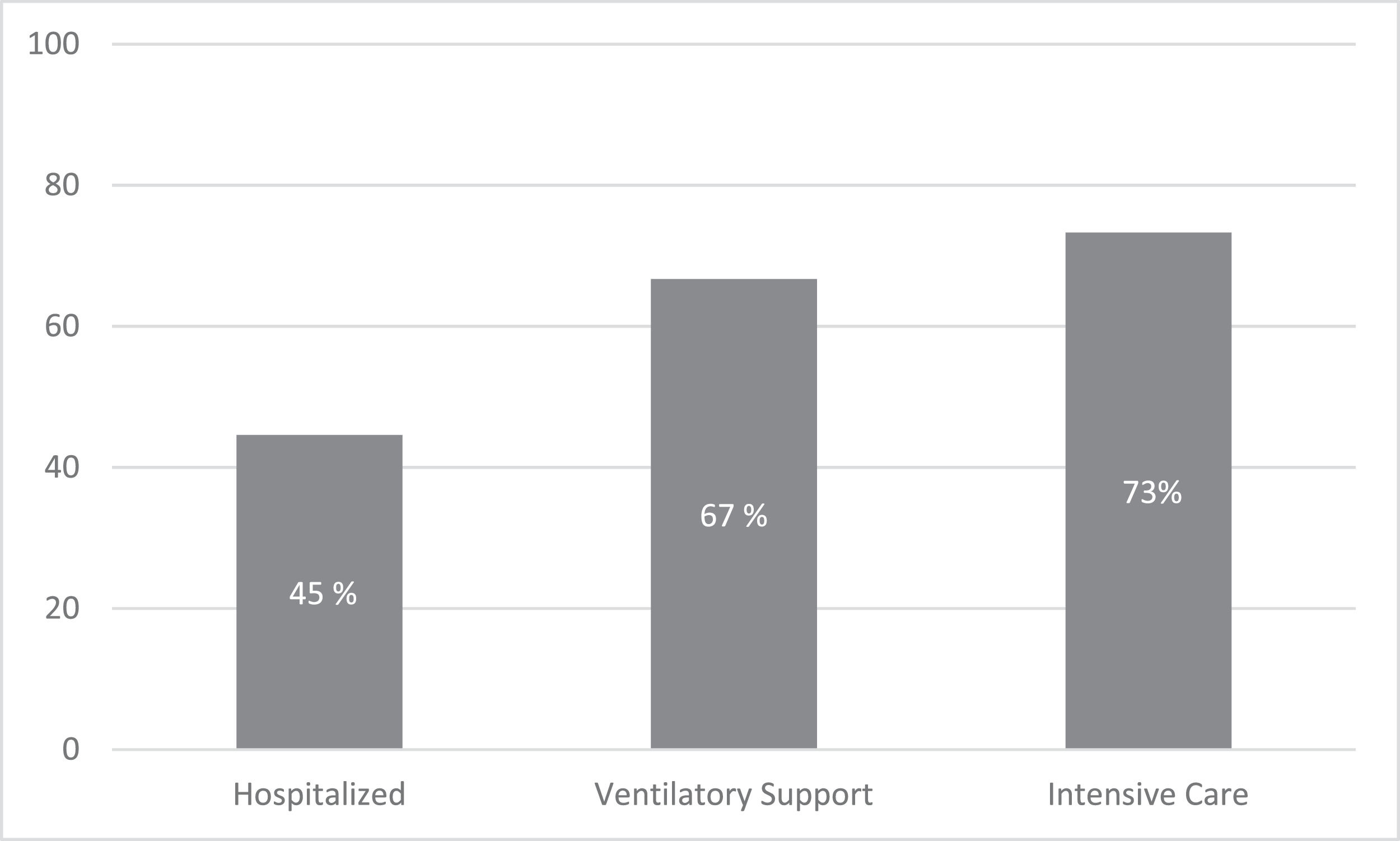
The overall mortality was 30%. Mortality rates were 45%, 67%, and 73% in hospitalized, ventilatory support, and ICU patients. (Figure 1) By univariate analysis, age (p=0.028), ECOG performance status (p=0.005), and MM therapy, including corticosteroids (p=0.015), were associated with increased mortality. (Table 5) By multivariate model, only ECOG performance status (OR 11.5; 95% CI 1.9 – 69) remained associated with mortality. (Table 6)
Characteristics of deceased or recovered patients.
Note: Percentages and statistics including only valid cases; NS: not statistically significant.
Multivariate analysis of clinical outcomes.
| Hospitalization | Ventilatory Support | ICU | Death | |||||
|---|---|---|---|---|---|---|---|---|
| Univariate p-value | Multivariate p-value (OR; 95%CI) | Univariate (p-value) | Multivariate p-value (OR; 95%CI) | Univariate (p-value) | Multivariate p-value (OR; 95%CI) | Univariate (p-value) | Multivariate p-value (OR; 95%CI) | |
| Age group, n (%) | 0.008 | 0.045 (2.02; 1.02 – 7.7) | 0.023 | NS | 0.003 | NS | 0.028 | NS |
| ECOG status | - | - | - | 0.005 | 0.008 (11.5; 1.9 – 69) | |||
| Hypertension | 0.014 | 0.016 (4.5; 1.3 – 15.5) | - | - | - | |||
| Previous SCT | 0.025 | NS | - | - | - | |||
| Corticosteroids* | 0.028 | 0.015 (4.3; 1.3 – 14) | 0.006 | 0.011 (5.1; 1.4 – 18) | 0.015 | NS | ||
| Immunomodulatory drugs* | 0.07 | NS | 0.028 | NS | 0.008 | 0.031 (3.4; 1.1 – 10) | 0.09 | NS |
| Monoclonals * | 0.025 | NS | 0.002 | 0.003 (5.7; 1.8 – 18) | 0.015 | NS | ||
Regarding the 60 patients who recovered from COVID, 38 (63%) had the current MM treatment delayed. Patients who did not require hospitalization had a delay in MM treatment in 55% of cases. Those who recovered after hospitalization, ventilatory support, or ICU support had 68%, 91%, and 100% of treatment delays.
DiscussionIn this series, MM patients diagnosed with COVID-19 had a very high frequency of hospitalization, ventilatory support requirements, ICU admission, and deaths. Although not associated with increased mortality, the therapy regimen was associated with severity condition. There was a high frequency of MM treatment delay in COVID-19 recovered patients.
MM is a disease with severely impaired immunity, including B-cell dysfunction leading to hypogammaglobulinemia, T-cell, dendritic cell, and NK cell abnormalities.7 Infection complications are frequent at diagnosis and remain clinically significant during all phases of treatment.1,2,8
A significant impact of the COVID-19 pandemic on MM patients had been expected since the first report of cases, as viral infections constitute most infections in patients with MM, and the respiratory tract is the most common site.9
The Spain Cooperative Group provided the outcomes of a large national cohort of MM patients hospitalized with confirmed SARS-CoV-2 infection. Regarding their data, MM patients had mortality rates 50% higher than noncancer patients with COVID-19.10 In a report from a single center, including patients with COVID-19 managed as in and out-patient, the infection course was most moderate or severe, with 56% requiring hospitalizations, 20% of intensive care, and 18% of deaths.11 The in-patient mortality of both studies was around 30%-35%. Our patients required more often hospitalization and a significantly higher in-patient mortality rate (45%); three in four cases requiring ICU support were deceased in our cohort.
ECOG performance status was our cohort's most critical risk factor for mortality. ECOG performance status is a validated fragility score and an outcome predictor in cancer patients, independent of age and comorbidities index. The other COVID cohorts in MM patients had also addressed age, renal disease, and active/progressive disease as predictors of COVID outcome.11,12
Although none of the prior MM treatments significantly influenced the mortality rate in our cohort, they were associated with the severity of the infection. Corticosteroids and monoclonal antibodies increased rates of ventilatory support, and corticosteroids and immunomodulatory drugs were associated with ICU admission.
The MM treatment plays a role in the risk of overall infection, and patients with at more lines of therapy have an increased risk of infection due to cumulative immunosuppression.8,9 Corticosteroids increase the risk of all types of infections through various mechanisms, and the risk of infection is directly proportional to the dose of glucocorticoid use. In our cohort, patients treated with combinations including corticosteroids required more ventilatory support and intensive care.
Monoclonal antibodies, in our cases, represented by Daratumumab (anti CD38 monoclonal antibody), are associated with the reactivation of latent viral infections, neutropenia, and pneumonia. Some studies reported high frequencies of severe COVID-19 in MM patients under anti-CD38 treatments.13,14 Its mechanism of action can explain the association with the severity of COVID in our cohort.
At last, immunomodulatory drugs were associated with ICU admission in our cohort. IMiDs downregulate the transcription factor PU.1, which disrupts granulocyte differentiation and induces neutropenia, resulting in an increased risk of severe infection at all stages of treatment.8,15 Another critical point is the association between thrombosis and IMiDs. As in COVID patients, thrombosis is a concern, and special attention is required.
There were a limited number of breakthrough infections after COVID-19 vaccination in our study. However, as the cohort started before a massive vaccination program in Brazil, no associations can be made. Vaccination is crucial in this population, but seroconversion is lower in MM patients compared to the general population and patients remain at risk of breakthrough infections even after complete vaccination.16-18
Our results showed that more than half of patients who did not required hospitalization had delayed MM treatment. The rate increases to 100% if the patients recover after ICU admission. An extended follow-up is necessary to evaluate if it could modify the outcome of these patients who survived.
A recent study evaluated the potential changes of the COVID pandemic in the treatment course of MM patients. Analyzing MM patients from more than 280 cancer centers in the US, they noted that patients with MM diagnosis performed during COVID were less likely to initiate treatment than those diagnosed before the pandemic.5 Studies in other cancer patients' subgroups also reported modifications in the patient's care during COVID.10-23
Our study has several limitations. Due to the unequal access to healthcare in our country, which was intensified by the pandemic, our cases may not be representative of the national reality since only cases reported by GBRAM centers were included. Patients from other centers could have had worse outcomes than those reported. Additionally, none of our patients received antiviral drugs such as remdesivir, Paxlovid, or molnulpiravir, despite their approval by the Brazilian regulatory agency (Remdesivir was approved in 2021, and others in 2022). Until now, access to treatment has been challenging, even for immunocompromised patients.
On the other hand, the incorporation of various COVID-19 vaccines with different platforms into the national immunization program in Brazil has led to a massive vaccination, and most patients having heterologous vaccine schedules. This variability makes assessing immune status more challenging.
The COVID-19 pandemic has dramatically impacted health care globally, but its impact on managing hematologic malignancies in low-income nations is an even higher challenge. We found that our patients were at the most significant risk of mortality from COVID-19 compared with MM patients from other reports. The increase in mortality among our patients was associated with performance status, but the treatment regimen was associated with disease severity. Most cases had the MM treatment at least delayed, and it needs special attention as it can result in long-term prognosis. These data are not surprising and emphasize the frailty of MM patients in Brazil. To limit severe events in this myeloma setting, close monitoring, optimal vaccination strategies, and access to treatment remains highly required, particularly in elderly patients with co-morbidities.
Authors' contributionMG, EQC, GR, AM, and VH were responsible for the conception and design of the study, conducting the search, analysis, and interpretation of data, and drafting the article. EQC, GR, VH, RB, RJPM, KRZ, AEN, JSL, CBS, EGS, and AMF were responsible for data acquisition and provided feedback on the manuscript. All authors reviewed and approved the version to be submitted.