Chimeric antigen receptor T (CAR-T) cell therapy is an emerging treatment option for relapsed/refractory multiple myeloma (RRMM) that is a multi-step process involving various stakeholders. Appropriate education on the practical logistics is therefore paramount to ensure treatment success.
MethodsA group of key opinion leaders met to explore the key elements of setting up and running a CAR-T center in Brazil. For each step in the CAR-T cell therapy process, the experts agreed on basic requirements, gave their key recommendations from practical experience, and considered any remaining unanswered questions.
ResultsThis paper presents best-practice recommendations and advice on how to overcome common challenges for each step in the CAR-T cell therapy process, with a focus on the current situation in Brazil. Key themes throughout the process are collaboration within the multidisciplinary team and with the referring physician, along with communication and education for patients and their caregivers.
ConclusionWe believe that the expert insights presented in this paper, in particular on optimal patient selection and timing of CAR-T cell therapy, will deepen understanding of the CAR-T process and aid implementation of this novel therapy for patients with RRMM in Brazil.
Multiple myeloma (MM) is a plasma cell malignancy accounting for 1–2% of all cancers.1 The treatment landscape for MM has significantly changed in recent years with the availability of proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and anti-CD38 monoclonal antibodies (mAbs).2 This has led to significant improvements in disease control, but cure remains elusive, and the majority of patients still relapse and become refractory to different treatments throughout their disease course.2 Therefore, there is an unmet need for novel treatment options in the relapsed/refractory (RR) setting, especially for triple-class refractory patients (patients refractory to a PI, IMiD and anti-CD38 mAb) where outcomes remain poor.3
Chimeric antigen receptor T (CAR-T) cell therapies, which combine antigen recognition and T-cell signaling domains,1 are an emerging treatment option for hematological malignancies, with six different CAR-T products approved by the FDA.4,5 Recently, ANVISA has approved two CAR-T cell therapies in Brazil: tisagenlecleucel for pediatric patients and young adults with RR B-cell acute lymphoblastic leukemia (ALL) and adults with RR diffuse large B-cell lymphoma,6 and ciltacabtagene autoleucel (cilta-cel) for patients with RRMM.7
B-cell maturation antigen (BCMA) is a promising target for drug development in MM. It is restrictively expressed on plasma cells and essential for myeloma cell survival and proliferation.8 Currently, two BCMA-directed CAR-T cell therapies, idecabtagene vicleucel (ide-cel) and cilta-cel, are FDA approved.4,9 Ide-cel was investigated in patients with RRMM in the pivotal Phase 2, single arm KarMMa trial (NCT03361748).10 In all treated patients, overall response rate (ORR) was 73.0%, with 33.0% of patients achieving at least a complete response (CR).10 Median progression-free survival (PFS) was 8.8 months.11 Cilta-cel was investigated in the Phase 1b/2 CARTITUDE-1 trial (NCT03548207).12 After a median follow-up of 27.7 months, the ORR was 97.9%, with 82.5% of patients achieving stringent CR.13 The 27-month PFS and OS rates were 54.9% (95% C.I.: 44.0–64.6) and 70.4% (95% C.I.: 60.1–78.6), respectively; median PFS and OS were not reached.13 Although ide-cel and cilta-cel have shown deep responses in heavily pre-treated patients, they are associated with predictable toxicity, with 84% and 95% of patients experiencing cytokine release syndrome (CRS) in the KarMMa and CARTITUDE-1 trials, respectively.10,12 Fortunately, the majority of CRS events were Grades 1 and 2.10,12
CAR-T cell therapy is a multi-step process involving numerous stakeholders requiring close coordination; appropriate training of healthcare professionals (HCPs), patients and caregivers is therefore essential.14 It is also important to consider optimal timing of CAR-T cell therapy along the MM patient treatment journey. Initially, CAR-T cell therapy can only be delivered at certain authorized centers,15 so close coordination between these centers and referring centers is essential.14 An educational toolkit on the implementation of CD19-directed CAR-T cell therapies has been previously published.14 There is currently an unmet need for an educational toolkit focused on CAR-T cell therapies for the treatment of MM.
As CAR-T cell therapy for MM moves from a clinical trial setting to real-world practice, it is important that resources are available to facilitate the operation of new CAR-T centers and multidisciplinary teams. This paper presents advice and expert recommendations from key opinion leaders (KOLs) with practical experience in delivering CAR-T cell therapy, and MM experts, with a focus on circumstances in Brazil. The objective of this paper is to provide education and best-practice recommendations to guide and standardize patient care across Brazil at each stage of the CAR-T cell therapy process.
MethodsA group of KOLs met for a round-table advisory exchange to explore the key elements of setting up and running a CAR-T center. A hematologist and the CAR-T coordinator based at the University of Navarra, a center with extensive practical experience of delivering CAR-T cell therapy in clinical trials, provided their expert advice. Topics discussed were accreditation, patient eligibility and referral, leukapheresis, bridging therapy, lymphodepletion, infusion and patient monitoring. Questions were posed by hematologists from Brazil who have expertise in MM, with a focus on applying these recommendations to the Brazilian healthcare system.
For each step in the CAR-T cell therapy process, the experts gave their key recommendations from practical experience, and considered any common challenges and how to overcome these. Using specialist knowledge of their healthcare system, the Brazilian hematologists considered specific practical information regarding the implementation of these recommendations in Brazil.
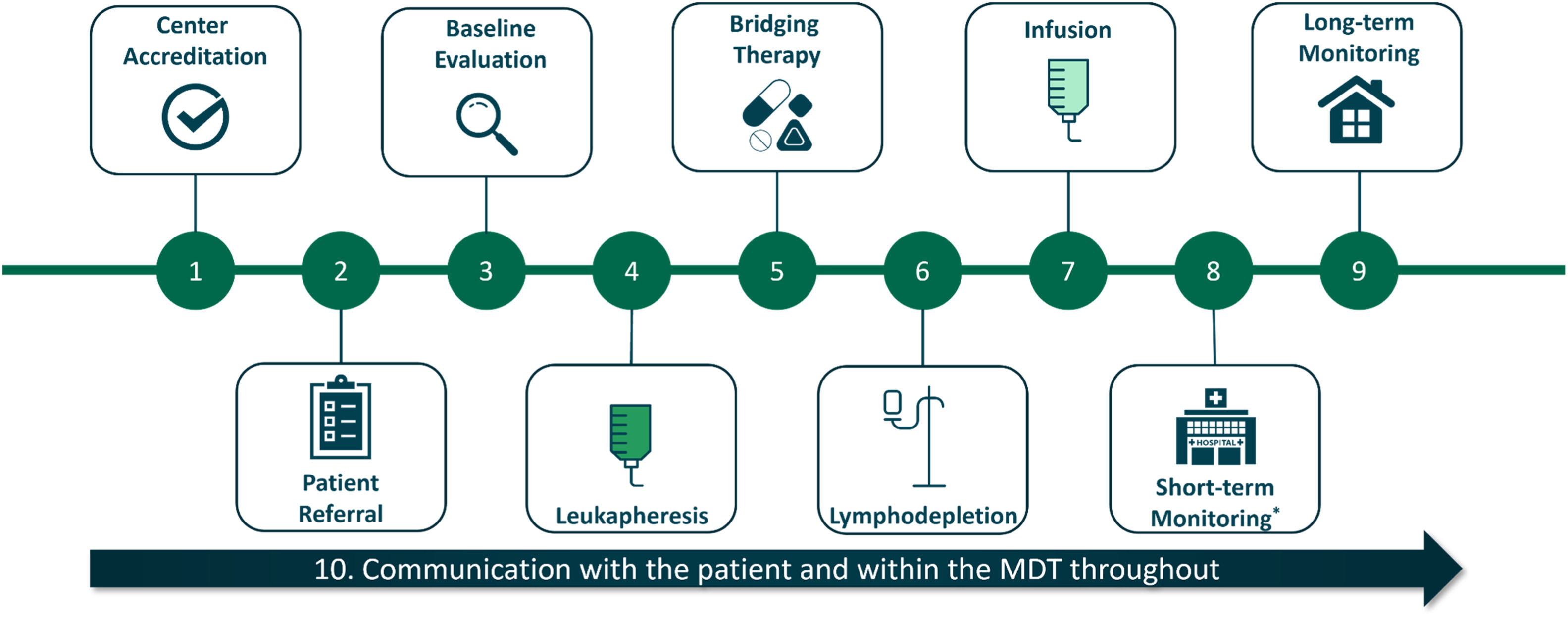
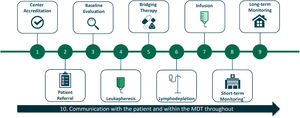
ResultsFigure 1 summarizes the key steps involved in the practical implementation of CAR-T cell therapy. Best-practice recommendations and common challenges are discussed for each step below.
1. Center accreditation
Healthcare centers must be validated and accredited before carrying out commercial CAR-T cell therapy.15 Assessing committees for cell therapy in Brazil are the American Association of Blood Banks/Brazilian Association of Hematology, Hemotherapy and Cellular Therapy (AABB/ABHH) and the Foundation for the Accreditation of Cellular Therapy-Joint Accreditation Committee, ISCT/EBMT (FACT-JACIE).16 Standard operating procedures must be in place for every step of the CAR-T process, and continuous training provided to all relevant staff.
Best-practice recommendations
- •
While FACT-JACIE accreditation is no longer a requirement for CAR-T cell therapy, such accreditation will speed up the validation process carried out by the manufacturer, and help ensure the site maintains all required quality processes.
- •
A multidisciplinary team of experts should be set up to manage the CAR-T process and discuss patient cases. The members of this team may vary, but should include hematologists, neurologists, intensive care unit (ICU) specialists, infectologists, pharmacists, nurses, transfusion specialists and a CAR-T coordinator. It can be useful to include cardiologists.
Overcoming common challenges
The accreditation process can be lengthy (18–24 months), so centers should prepare for this step in advance.
2. Patient eligibility and referral
Patient eligibility will be based on product labels and patient characteristics. Ide-cel and cilta-cel are currently FDA approved for the treatment of adult patients with RRMM after 4 or more prior lines of therapy, including an IMiD, a PI and an anti-CD38 mAb.4,9 In Brazil, ANVISA has approved cilta-cel for patients previously exposed to a PI, an IMiD and an anti-CD38 mAb, with no restrictions on the number of prior lines of therapy.7
Best-practice recommendations
- •
Choosing the right patient for CAR-T cell therapy is one of the most important steps in the CAR-T process to maximize benefits, while reducing the number of patients who become ineligible between leukapheresis and infusion. In order to avoid the referral of non-eligible patients, referring physicians should be properly educated on and have a checklist for eligibility requirements. In addition, advanced planning is required to accommodate the time for each step of the process; this should be considered during patient selection.
- •
Requirements for eligibility include:
- ○
Patient-related factors
▪Frailty/level of disability: Frailty should be considered rather than age when assessing eligibility for CAR-T cell therapy.5 There is not a standardized frailty score for assessing patients, but various scores such as the International Myeloma Working Group (IMWG) score or the simplified frailty score may be used.17,18 There are currently limited data on the effects of CAR-T cell therapy in elderly patients; however, subgroup analyses of the KarMMa and CARTITUDE-1 trials have shown comparable responses in older patients to other subgroups.13,19
▪Comorbidities: There must be careful consideration of risk-benefit ratio when deciding to treat patients with comorbidities.
▪Eastern Cooperative Oncology Group Performance Status (ECOG PS): An ECOG PS of 0–1 is recommended for optimal outcomes.5
▪Adequate cardiac, respiratory and renal function: Fludarabine, which is required as part of the lymphodepleting regimen to maximize CAR-T cell expansion,15 should not be administered to patients with creatinine clearance <30 mL/min.20 Dose reductions of cyclophosphamide and fludarabine may be considered depending on the patient's renal function.5 Cardiac and respiratory function are also important and should be evaluated.
▪Caregiver availability: Patients need the assistance of a dedicated caregiver throughout their treatment.14
▪Logistical considerations: Patients may be required to travel to the nearest authorized CAR-T center for treatment and remain there for monitoring for at least 2 weeks post-infusion.5 Patient preference and willingness to travel should be considered.
- ○
Disease-related factors
▪Product labels: Patients must fit the indication on the product label, including exposure to previous therapies and refractoriness.
▪Progression of the disease: Disease should be sufficiently stable that patients remain in good condition to receive their infusion after the manufacturing period. A life expectancy of more than 6–8 weeks is recommended by the European Society for Blood and Marrow Transplantation.5
▪Tumor burden: High tumor burden has been related to an increased risk of CRS and decreased CAR-T cell efficacy in patients with RR B-cell ALL.21
▪Difficult-to-treat patient populations: We do not recommend that patients with high-risk cytogenetic features or extramedullary disease should be excluded from CAR-T treatment, but careful consideration of the risk-benefit ratio is required. A subanalysis of the KarMMa trial in difficult-to-treat patients concluded that deep and durable responses were achieved in most subgroups of patients, even those with aggressive disease features (i.e. extramedullary disease and high-risk cytogenetics).22 Most patients in high-risk subgroups also responded in the CARTITUDE-1 trial, suggesting that CAR-T cell therapy offers greater efficacy in high-risk patient populations than currently available treatments.13
- ○
- •
When centers first begin delivering CAR-T cell therapy, they should use experience from clinical trials and select patients who stringently fit the product indication. Careful evaluation is required in specific patient populations, especially as centers build their experience. According to the experts’ opinion based on practical experience, patient populations who require a careful evaluation are those with aggressively progressing disease, high levels of lactate dehydrogenase, plasmacytomas located in risky places, prior history of cast nephropathy at the time of relapse, or limited bridging therapy options.
- •
It is important to preselect potential candidates (based on disease and patient characteristics) while on salvage therapy and begin communication with a CAR-T center to ensure rapid referral. Pre-eligibility checks can also take place before referral to the CAR-T center.
Overcoming common challenges
In the KarMMa and CARTITUDE-1 trials, a subset of patients underwent leukapheresis but did not receive the CAR-T cell infusion (8.6% and 14.0% of patients, respectively).10,12 Rapid referral of patients can minimize this in real-world practice.
Referring physicians should be aware that the eligibility criteria for autologous stem cell transplant (ASCT) and CAR-T differ; while patients >70 years are unlikely to be eligible for ASCT, age is deemed less important for CAR-T cell therapy.5 Targeted education on this topic will ensure that potentially eligible patients are not overlooked because of their age.
While established risk scores exist for ASCT,23,24 there are not yet equivalent scores available for CAR-T cell therapy. HCPs should therefore be aware of eligibility requirements for CAR-T cell therapy (listed above) in order to carefully consider the risk-benefit ratio of treatment for each patient.
3. Baseline evaluations
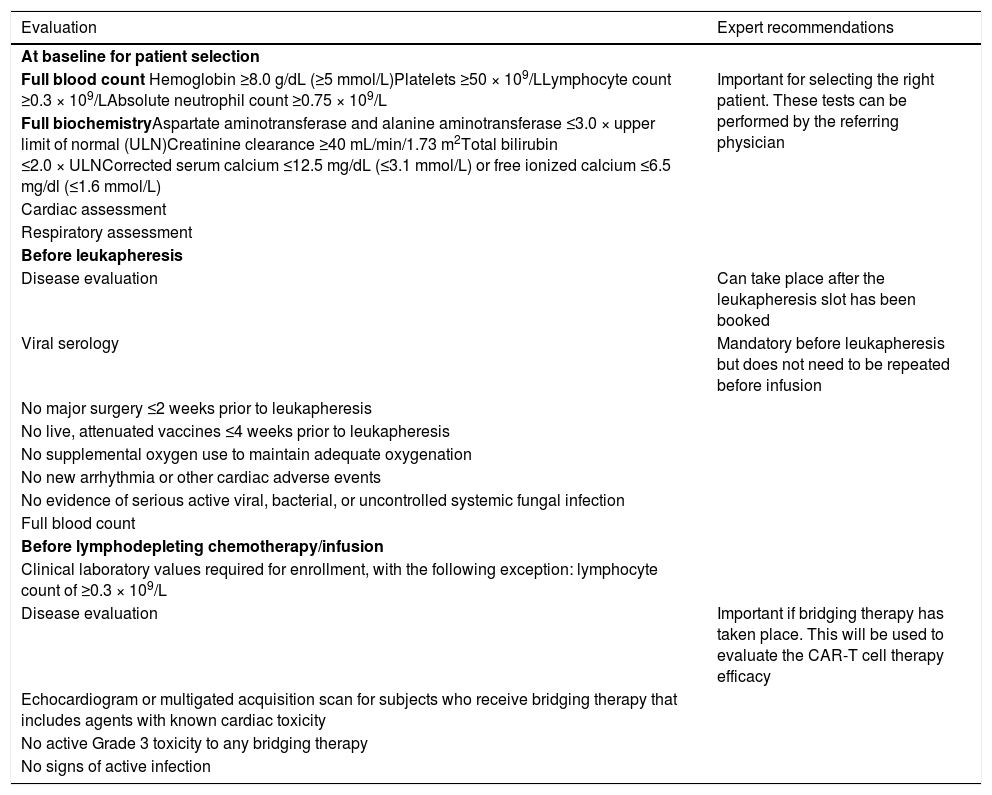
There are several checkpoints throughout the CAR-T cell therapy process where patients must be evaluated for their eligibility (Table 1). Should a patient fail these baseline evaluations, they will not be able to progress to CAR-T cell therapy.
Recommended evaluations throughout the process.
Best-practice recommendations
- •
Baseline evaluations can take place with the referring physician or at the CAR-T center; however, the CAR-T center may choose to perform a new set of evaluations upon referral.
- •
A general neurological assessment should be carried out prior to leukapheresis, with a more in-depth evaluation carried out before lymphodepletion (Section 6).
Overcoming common challenges
Cytopenias can be present in patients with heavily pre-treated RRMM. This can compromise eligibility for leukapheresis, and therefore CAR-T cell therapy. Adequate washout periods are required before leukapheresis to allow recovery from cytopenias.5 Growth factor therapy can be considered at the physician's discretion.
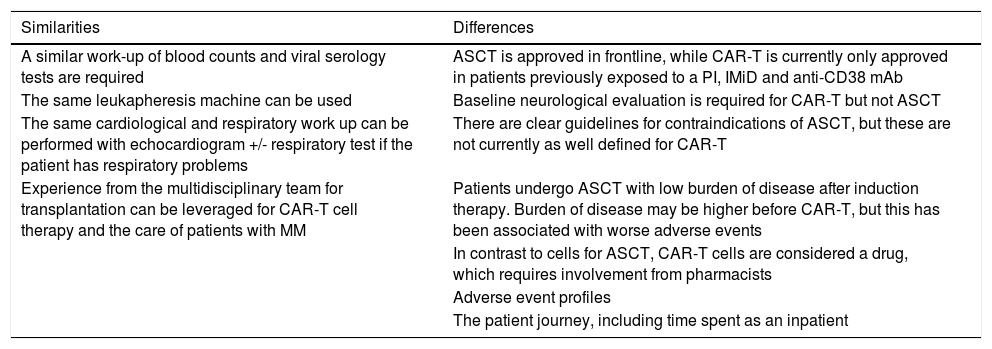
4. Leukapheresis
Leukapheresis for commercial CAR-T products in Brazil must take place in certified centers. Many centers will have experience delivering ASCT and can leverage this when starting to deliver CAR-T cell therapy. Table 2 lists similarities and differences between ASCT and CAR-T cell therapy.
CAR-T cell therapy and ASCT.
Leukapheresis, product order and shipment details should be coordinated with the manufacturer.5 This is often completed via an online order management platform; each company will likely use their own platform.
Best-practice recommendations
- •
A washout period for anti-myeloma therapies is required to ensure T-cell fitness. Washout periods of ≥7 days for PIs or IMiDS, and ≥14 days for corticosteroids or mAbs are recommended prior to leukapheresis. Further evidence is required to optimize these timings. It is recommended to avoid bendamustine or high dose alkylators as the last line of therapy before CAR-T cell therapy. A retrospective analysis of the KarMMa trial found that patients with more recent alkylator exposure had fewer T cells in their peripheral blood mononuclear cell material.25
- •
Prior to leukapheresis, patients should be evaluated for their eligibility (Table 1). At a minimum, these evaluations should include full blood counts, COVID-19 screening and serological testing for hepatitis B, hepatitis C, human immunodeficiency virus, cytomegalovirus, syphilis and human T-lymphotropic virus type 1. Serological results should be obtained within 30 days of leukapheresis.5
- •
Infection prevention and vaccine prophylaxis are important considerations at every stage of MM treatment. Patients should be immunized with inactivated vaccines only according to IMWG guidelines26 before starting leukapheresis. This should include vaccination against influenza, herpes zoster and all other indicated immunizations. Antimicrobial and antiviral prophylaxis should also be considered, as well as immunoglobulin replacement in certain patient populations.26 Follow local and professional-bodies issued guidelines for vaccination and prophylaxis against COVID-19, as advice is still evolving.
- •
After leukapheresis, manufacturers may request for the leukapheresis product to be shipped fresh or frozen to the manufacturing facility. If cryopreservation is carried out locally, then the center must be accredited in this process.
Overcoming common challenges
Once manufactured, CAR-T cells are classed as advanced therapy medicinal products. All members of the multidisciplinary team who receive or manage the CAR-T cells (including pharmacists) must therefore be appropriately trained.27
5. Bridging therapy
The aim of bridging therapy (therapies given in the 4–6 weeks it takes to manufacture the CAR-T cells) is to reduce disease burden and maintain performance status before infusion, thus improving the efficacy and safety of the CAR-T cells.5,15 In the KarMMa and CARTITUDE-1 trials, 88% and 75% of patients received bridging therapy during the manufacturing period, respectively.10,12 Commonly used bridging therapies as single agents or in combination were corticosteroids, PIs, alkylating agents and monoclonal antibodies.10,12 Bridging therapy can take place at the CAR-T center or the patient's local referring center. Communication between the two sites is therefore key.
Best-practice recommendations
- •
The choice of bridging therapy will be patient specific, based on prior exposure and toxicity. This decision should be made in collaboration with the referring physician.
- •
Some considerations include:
- ○
Therapies that increase risk of infection or cytopenia should be avoided.
- ○
Consider radiotherapy for extramedullary disease.
- ○
Consider avoiding BCMA-targeted therapies (e.g., antibody-drug conjugates or bispecific antibodies) prior to treatment with BCMA-directed CAR-T cell therapies to prevent possible downregulation of BCMA and reduced efficacy of CAR-T cell therapy.
- ○
- •
In the KarMMa and CARTITUDE-1 trials, bridging therapies were restricted to therapies that patients had previously received or responded to.10,12 In contrast, therapies that patients are naïve to are preferred in the real-world setting.
- •
A washout period may be required before CAR-T cell infusion; the length of this will depend on the type of bridging therapy and product guidelines should be followed.
- •
Provide adequate prophylaxis to avoid infection complications that may impact infusion.
Overcoming common challenges
Close communication is required between the CAR-T and referring centers, as well as with the CAR-T cell manufacturer, to coordinate the timing of washout periods with the release of the CAR-T product.
Disease progression before infusion could cause ineligibility for CAR-T cell therapy. If disease progression occurs, next steps should be discussed with the referring physician. The risk of progression between leukapheresis and lymphodepletion can be minimized by carefully considering patient eligibility (Section 2), and selecting the most appropriate bridging therapy on a case-by-case basis.
6. Lymphodepletion
A lymphodepleting regimen is administered to patients before infusion in order to maximize CAR-T cell expansion.5 A minimum of 2 rest days are required between completion of lymphodepletion and the CAR-T cell infusion.5
Adequate and extensive clinical evaluation is required to confirm patient eligibility for the lymphodepleting regimen and subsequent CAR-T cell infusion (as discussed in Section 2).5 Baseline evaluation of disease burden is also required before lymphodepletion,5 and is used to measure the response to CAR-T cell therapy (Table 1).
Best-practice recommendations
- •
A consultation with a neurologist, including baseline neurological tests (magnetic resonance imaging [MRI] and electroencephalogram [EEG]), is recommended for every patient in case of subsequent immune effector cell-associated neurotoxicity syndrome (ICANS).
- •
Cardiological assessments (transthoracic echocardiography, electrocardiogram and cardiac biomarkers) are required if cardiotoxic drugs have been used as bridging therapy.5
- •
The lymphodepleting regimen is generally well tolerated and can be delivered in an outpatient setting, although this should be assessed on a case-by-case basis.
- •
Prophylaxis for nausea and vomiting should be considered, but hydration is generally not required.
Overcoming common challenges
Lymphodepletion should be coordinated with CAR-T product release.
7. CAR-T cell infusion
The CAR-T product will be delivered back to the CAR-T center and appropriately stored until the patient is ready for infusion.4,9
Best-practice recommendations
- •
During the establishment of CAR-T cell therapy in Brazil, patients should be hospitalized for infusion and at least 14 days after for close monitoring. Outpatient infusion in Brazil is a future perspective for centers with extensive experience, and that have proper arrangements in place.
- •
Administer pre-medication per product guidelines to minimize the risk of infusion reactions (generally paracetamol and an antihistamine). Corticosteroids should be avoided.5
- •
Thaw the CAR-T cells as close to the patient as possible (preferably at bedside).
- •
CAR-T cells are infused through a central line. If the central line will be maintained for both leukapheresis and infusion then it is preferrable to use a high-flow bilumen and tunneled line. In the event that the line is not going to be maintained for both processes, then a non-tunneled line is recommended for infusion and management of possible complications afterwards.
- •
Consider prophylaxis for tumor lysis syndrome (especially for patients with high disease burden).
- •
Communicate to the ICU department that a patient is receiving CAR-T cell therapy in case of serious adverse events.
Overcoming common challenges
The manufacture of CAR-T cells and quality control steps are carried out at a central site and are not in the control of HCPs at the CAR-T center. Keeping in close communication with the manufacturer is essential.
8. Short-term monitoring
Patients should remain hospitalized for close monitoring in the first 14 days post infusion.5 Access to tocilizumab along with adequately trained ICU specialists are important requirements.28
The American Society for Transplantation and Cellular Therapy (ASTCT) Consensus Grading scale grades CRS on a scale of 1–4 based on fever, hypoxia and hypotension.29 The ASTCT ICANS grading scale incorporates the Immune Effector Cell-associated Encephalopathy (ICE) score with level of consciousness, presence of seizure, motor findings and elevated intracranial pressure/cerebral edema to grade ICANS on a scale of 1–4.29 Collaboration with trained neurologists is important for the proper monitoring and management of ICANS.
Best-practice recommendations
- •
Each CAR-T product will have a different adverse event profile; it is important to be aware of median times to adverse event onset with individual products when considering post-infusion monitoring. For example, median time to onset of CRS was 1 day (range 1–12) and 7 days (range 1–12) in the KarMMa and CARTITUDE-1 trials, respectively.10,12
- •
Fever is usually the first symptom of CRS, followed by hypoxia and hypotension; hypofibrinogenemia is also common.
- •
Tocilizumab should be given early to halt CRS progression (to all patients with ≥Grade 2 or persistent Grade 1 CRS).
- •
Hemophagocytic lymphohistiocytosis/macrophage activation syndrome can occur in patients following treatment with CAR-T cell therapies, potentially at the same time as CRS or neurologic toxicities. Daily communication with an ICU specialist is recommended in the case of serious adverse events.
Overcoming common challenges
There must be early communication with ICU specialists to educate them on CAR-T cell therapy.5 Some ICUs may be reluctant to admit patients at late lines of therapy with a low life expectancy. It is important to include ICU specialists in the multidisciplinary team for education on CAR-T cell therapy and discussions around patient eligibility.
9. Long-term monitoring
Long-term monitoring takes place once the patient has been discharged from the CAR-T center and should focus on disease status monitoring, infection prevention and supportive care for any long-term adverse events.14 Long-term care is a joint responsibility of the referring physicians and CAR-T centers. Over time, patients will be monitored more often by their referring physician than their CAR-T physician.14 Regulatory boards may mandate that patients who have received CAR-T cell therapy should be followed up for 15 years to understand the long-term effects of the therapy, including the risk of secondary malignancies due to insertional mutagenesis.5
Best-practice recommendations
- •
Carry out regular disease monitoring. The frequency of imaging for disease monitoring will depend on the patient's disease status.
- •
Carry out basic monitoring for cytopenias, infections and organ function.
- •
Patients should be screened for dysplasia if they have cytopenias, as they may have received alkylators in previous treatment regimens.
- •
Immunoglobulin replacement therapy may be necessary for some patients,30 and this should be managed in collaboration with the referring center.
- •
HCPs should be proactive in asking patients about their vaccination status; vaccinations should be repeated 3 months post infusion.
Overcoming common challenges
The transition from the CAR-T center back to their local center can be difficult for some patients. Rehabilitation, at-home nursing support or support from social workers may be necessary for some patients,14 and the multidisciplinary team should help to arrange this.
10. Communication
The organization of CAR-T cell therapy is the responsibility of a multidisciplinary team of experts.14 A CAR-T coordinator should lead this group and be responsible for organizing regular meetings. At these meetings, the multidisciplinary team should discuss the eligibility of referrals and the status of active patients in the center.
CAR-T cell therapy is a multi-step process, so appropriate patient education on each step is necessary.14 Patients and their caregivers must be educated on how to recognize adverse events. Caregivers play an important role in patient care post infusion,5,14 and are often the first to notice subtle changes in the patient's state.14 Caregivers are expected to stay with the patient at all times if care is delivered in an outpatient setting and should be educated on detection of adverse events and when to call for help.15
Best-practice recommendations
- •
Telemedicine can be useful for initial consultations with patients, especially in large countries like Brazil where patients may not live near a CAR-T center. Close communication between the CAR-T center and the referring physician is critical when patients live far from the CAR-T center.
- •
Nurses should be in frequent communication with patients and their caregivers to provide support and education.15
Overcoming common challenges
Poor communication between CAR-T and referring centers could be a barrier to a successful CAR-T program. Regular meetings between the multidisciplinary team and the referring center can help overcome this.
Communication, education and organization are key for a successful CAR-T program. This paper presents best-practice recommendations for CAR-T cell therapy with the aim to educate HCPs on the practical implementation of CAR-T cell therapy in Brazil.
The strength of our recommendations is that they have come from practical experience in delivering CAR-T cell therapy at a center with extensive experience in the treatment of patients with RRMM (The University of Navarra), and consider common questions Brazilian HCPs may have about the implementation of CAR-T in real-world practice. Potential weaknesses of this paper are that recommendations have come from a single center, where most experience with CAR-T has been in a clinical trial setting. However, we feel that the recommendations made will be directly relevant to HCPs in Brazil, and beyond, who are involved in CAR-T cell therapy.
CAR-T cell therapy has demonstrated enormous potential in shaping the treatment landscape for patients with RRMM and significantly improving outcomes. We believe that the recommendations presented in this paper will help to educate HCPs and guide patient care across Brazil to make the most of this promising new therapy.
Medical writing support for the development of this manuscript, under direction of the authors, was provided by Sally Vanden-Hehir, PhD, of Ashfield MedComms, an Inizio Company, and was funded by Janssen.