The stiff person syndrome (SPS) is a rare and disabling neurological disorder characterized by muscle stiffness, painful spasms and rigidity involving the proximal and axial limb muscles, with an estimated incidence of 1 case per million per year. The first line of treatment for symptomatic management includes gamma-aminobutyric acid (GABA)ergic agonists, benzodiazepines and baclofen. The therapeutic plasma exchange (TPE), alone or as an adjuvant to other forms of immunomodulation, has been used as a therapeutic option, particularly in refractory cases.
MethodsAn observational study was performed to review SPS patient symptoms, comorbidities, electromyography (EMG) studies and treatment, identifying autoantibodies, therapeutic plasma exchange (TPE) procedural details and clinical response.
Main resultsFive patients (4 male and one female) were treated with TPE during the study period as adjuvant therapy. The average age was 47 years (range 34 - 61 years), and anti-glutamic acid decarboxylase 65-kilodalton isoform (anti-GAD65) antibodies were positive in 80 % (4/5) of the patient population. All patients received immunosuppressive drugs along with TPE. Four patients received TPE during the first admission and one received it during the third hospital admission. All patients showed good improvement immediately after TPE, but it was not a sustainable effect.
ConclusionTPE may be helpful as adjuvant therapy for SPS patients to provide relief from clinical symptoms
The stiff person syndrome (SPS), with an estimated incidence of 1 case per million per year, has a predilection for middle-aged individuals, preponderantly female. It is a rare and disabling neurological disorder characterized by muscle stiffness, painful spasms and rigidity involving the proximal and axial limb muscles.1-3 The diagnosis of SPS is based on recognizing clinical signs and symptoms (modified Dalakas criteria), supported by electromyography (EMG), antibody testing and response to diazepam.4 SPS is frequently observed with other autoimmune disorders, such as Graves' disease, pernicious anemia, vitiligo, diabetes and hypothyroidism. Glutamic acid decarboxylase (GAD) antibodies are present in approximately 85 % of the patients.4 The impairment of gamma-amino butyric acid pathways (GABAergic pathways) and reduced GABA levels result in characteristic clinical manifestations. The exact role of autoantibodies in the pathogenesis of SPS is still unclear. A few autoantigens have been identified in the inhibitory synaptic pathway. The presynaptic autoantigens include GAD and amphiphysin and the postsynaptic autoantigens include GABA receptor-associated protein (GABARAP) and gephyrin.3,4 The early recognition and diagnosis of SPS are important to initiate effective treatment and reduce morbidity. The treatment of SPS consists of GABA-enhancing drugs, anti-spasticity drugs and immunomodulating therapies.
The first line of treatment for symptomatic management includes GABAergic agonists, benzodiazepines and baclofen.4 Intravenous immunoglobulin (IVIg) treatment is also effective for clinical improvement.5 Therapeutic plasma exchange (TPE), alone or as adjuvant to other forms of immunomodulation, has been used as a therapeutic option, particularly in refractory cases.1 The TPE efficacy is unknown due to the absence of randomized controlled trials and only a few case reports with both positive and negative clinical outcomes are on record. As per the American Society for Apheresis (ASFA) 2023 guidelines, SPS is a category III indication for TPE.6 As a tertiary care referral center specializing in neurological, neuropsychiatric disorders and autoimmune diseases of the nervous system for a large proportion of cases that seek treatment here, we present our experience of the utility of TPE in patients with SPS.
Material and methodsAn observational study was performed to review the patient medical records diagnosed as SPS who underwent TPE between January 2015 and December 2022. The patient demographic details, symptoms, comorbidities, EMG studies, treatment, identified autoantibodies, TPE procedural details, clinical response and available follow-up in medical records were recorded and analyzed. A scoring system (a numeric 1 to 3 severity score) as described earlier by Pagano et al. evaluated the clinical improvements using TPE and other concurrent immunomodulation.1 The Institute Ethics Committee approved the study.
TPEAll TPE procedures were performed using peripheral venous access on the Haemonetics MCS plus 9000 cell separator (Haemonetics, Braintree, MA, USA). Each patient received a minimum of 5 TPEs on alternate days and approximately 30 to 40 ml/kg of plasma was removed during each cycle. On average, 5 to 7 cycles of TPE were performed. A combination of crystalloid (0.9 % normal saline) and colloids [hydroxyethyl starch (HES) and fresh frozen plasma (FFP) was used as replacement fluid.
ResultsPatient and clinical characteristicsThe study included five patients (4 males and one female). The demographic details, clinical symptoms, EMG findings, treatment and response are included in Table 1.
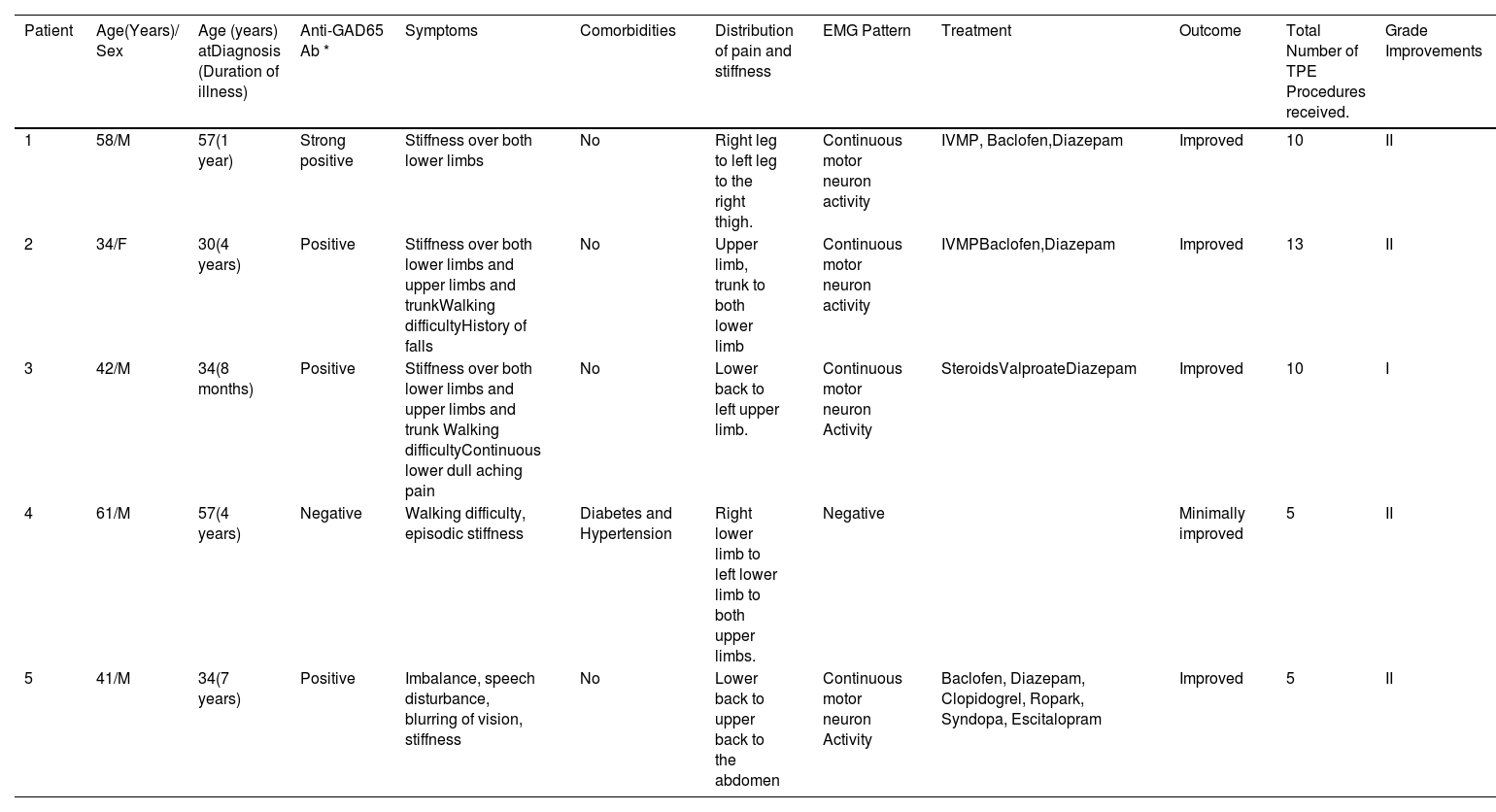
Details of patients who underwent TPE at our center.
| Patient | Age(Years)/ Sex | Age (years) atDiagnosis (Duration of illness) | Anti-GAD65 Ab * | Symptoms | Comorbidities | Distribution of pain and stiffness | EMG Pattern | Treatment | Outcome | Total Number of TPE Procedures received. | Grade Improvements |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58/M | 57(1 year) | Strong positive | Stiffness over both lower limbs | No | Right leg to left leg to the right thigh. | Continuous motor neuron activity | IVMP, Baclofen,Diazepam | Improved | 10 | II |
| 2 | 34/F | 30(4 years) | Positive | Stiffness over both lower limbs and upper limbs and trunkWalking difficultyHistory of falls | No | Upper limb, trunk to both lower limb | Continuous motor neuron activity | IVMPBaclofen,Diazepam | Improved | 13 | II |
| 3 | 42/M | 34(8 months) | Positive | Stiffness over both lower limbs and upper limbs and trunk Walking difficultyContinuous lower dull aching pain | No | Lower back to left upper limb. | Continuous motor neuron Activity | SteroidsValproateDiazepam | Improved | 10 | I |
| 4 | 61/M | 57(4 years) | Negative | Walking difficulty, episodic stiffness | Diabetes and Hypertension | Right lower limb to left lower limb to both upper limbs. | Negative | Minimally improved | 5 | II | |
| 5 | 41/M | 34(7 years) | Positive | Imbalance, speech disturbance, blurring of vision, stiffness | No | Lower back to upper back to the abdomen | Continuous motor neuron Activity | Baclofen, Diazepam, Clopidogrel, Ropark, Syndopa, Escitalopram | Improved | 5 | II |
A fifty-eight-year-old male patient presented (March 2020) with a history of intermittent stiffness and pain in the right lower limb that progressed to involve both lower limbs with increased frequency (5–6 times/day). He could not stand from the resting position, needed support for walking and had increased sensitivity to touch and noise. His autoimmune profile and malignancy screening with a PET scan were negative and his thyroid profile was normal. The patient was treated symptomatically with Baclofen, Diazepam and muscle relaxant. The TPE was initiated, with five procedures on alternate days, followed by five doses of intravenous methylprednisolone (IVMP) daily. The patient showed improvement in the form of reduced stiffness and pain. He could sit without support and walk with minimal support at discharge. The patient was prescribed 20 g/ day IVIg for five days and 1 gm/day IVMP for five days for the next five months, which he received at a local hospital. He showed good improvement (MRC Grade III) at six months of follow-up (September 2020). He could walk independently, with no spasms and minimal stiffness and resumed his office (December 2020).
After seven months (July 2021) of the asymptomatic period, the patient developed stiffness and a dragging gait in the right limb. He was given symptomatic treatment with Baclofen, Diazepam, five procedures of TPE and two doses of IVMP (120 gm). He improved, with reduced spasticity and could walk independently at discharge. In further follow-up (January 2022), his lymphocyte count (CD19 and CD20) was measured and had decreased. He received the first injection of Rituximab (500 mg) in two divided doses after one month of discharge, followed by a similar dose in the next month with a reported decreased stiffness. The patient was advised to continue the treatment and plan for TPE, but was lost in the follow-up.
Case 2A thirty-four-year-old female patient (April 2019) had a history of frequent falls initially four years previously, followed by stiffness of lower limbs and trunk, resulting in difficulty in walking. She was treated with steroids before visiting us and reported improvement. The symptoms gradually increased in severity and she could not stand and walk without support. She underwent seven procedures of TPE and IVMP and her pain subsided. She still had minimal stiffness in her legs, which sometimes caused difficulty in walking immediately after the TPE. However, she could walk independently at discharge (MRC GRADE II). She was on regular follow-up with monthly TPE, daily Baclofen and Diazepam for the first four months, followed by a monthly TPE procedure for the subsequent three months. She showed good improvement (December 2019) in her symptoms (GRADE III). The clinical team reduced the TPE frequency from monthly to quarterly. However, due to the ensuing Coronavirus-19 (Covid-19) pandemic, she could not attend the subsequent hospital TPE procedures. After two years (October 2021), she came for a follow-up and presented with aggravated symptoms in the last 3 to 4 months. Her autoimmune profile/thyroid profile/any malignancy were negative. The anti-GAD was strongly positive. The patient was started on a diazepam 10 mg tablet (four times a day, daily) for anxiety and depression along with a baclofen tablet (10 mg, BD) and discharged with a plan to initiate the TPE and rituximab if symptoms persisted. The symptoms improved with medication in the six-month follow-up (May 2022) and drug doses were further reduced. The subsequent three-month follow-up reported improvement in existing symptoms with no new symptoms.
Case 3A forty-two-year-old male patient (July 2017), a tailor by occupation, fell by tripping on trivial objects eight months previously. Subsequently, backache and stiffness with continuous non-radiating pain increased with sitting and bending and progressed to stiffness. He could not bend forwards, nor get up from the sitting position. He experienced worsening symptoms while walking and bending. The patient was treated with diazepam, baclofen and steroids and experienced significant improvement (GRADE III) after 18 months of follow-up (December 2018) with a GAD antibody reduction below detectable levels. The steroid was gradually tapered and stopped, while baclofen and diazepam were continued. The stiffness reappeared in the 22nd month (April 2019) (i.e., four months after stopping oral steroids). Repeat testing for GAD in the 30th month (March 2020) was still negative. In the 32nd month (June 2020), the condition worsened, with the patient reporting difficulty in daily living activities. He gradually developed difficulty turning on the bed with low backache and stiffness. The patient also developed fearfulness, anxiety and restlessness upon hearing sudden noises like a phone ringing. A repeat GAD test was positive. He was readmitted and treated with oral/IV - steroid, baclofen, diazepam and five procedures of TPE on alternate days. He was discharged with improved symptoms and advised to participate regularly in a follow-up with teleconsultation and email communication because of the Covid-19 pandemic.
After the first wave of the COVID-19 pandemic, the patient came for a follow-up (October 2020). He presented with a reappearance of symptoms and was hospitalized. He was started on a weekly wysolone 20 mg tablet and a weekly methotrexate 12.5 mg tablet. His symptoms improved and he was discharged with a plan for plasma exchange. He completed another five plasma exchange procedures (one session/week) on an outpatient basis along with medications. His symptoms were reduced, providing an improved quality of life. The patient complained of an increase in symptoms (increase in stiffness) with increased frequency (1/2 episodes per day) in the nine-month follow-up (August 2021). He continued the same treatment.
Furthermore, after three months of follow-up (October 2021), the frequency (1/2 episodes per day to 8/9 episodes per day) and intensity of the symptoms increased. He required support to walk. The patient experienced increased stiffness, behavior changes upon hearing loud noises, irreverent talking episodes, seizures, anxiety, palpitation and sweating. As the anti-GAD antibody was negative, the patient was started on oral medication (weekly wysolone 20 mg tablet Od, baclofen 10 mg tablet Bd, diazepam 5 mg tablet 4 times/weekly methotrexate 12.5 mg/folate tablet and vitamin D3 tablet). The patient improved with a 50 % reduction in symptoms. He was better in symptoms (March 2022) and was on regular medication. In a recent follow-up (September 2022), he complained of increased para-spinal muscle stiffness and difficulty in arising from bed (requiring support) and was advised to continue the treatment. He is still in follow-up.
Case 4A sixty-one-year-old male patient (June 2019) had difficulty in walking for 4 to 5 years. The illness was insidious and progressive in the form of episodic stiffness of the right lower limb with a tingling sensation in the foot and difficulty in placing the foot on the floor. The patient used to walk bizarrely. The stiffness progressed bilaterally to the left lower and upper limbs and he was finally bedridden.
He was treated with symptomatic drugs, steroids and five procedures of TPE. He had considerable improvement and could walk with support (GRADE II). As the patient was seronegative for GAD antibodies, the clinical team did not consider 2nd line treatment using immunosuppression with IVIg. He was advised to continue using baclofen and oral steroids and having physiotherapy after discharge. The patient was symptomatically better for a month after discharge, but for unknown reasons, the patient discontinued physiotherapy at home. He died two months after discharge due to pneumonia.
Case 5A forty-one-year-old male patient (April 2021) had a seven-year history of imbalance, speech disturbance for the last five years, blurred vision for two years and stiffness for the previous three months. The stiffness was over the trunk, progressing from the lower back to the upper back, chest and abdomen. It lasted 10 to 15 min, exacerbated by emotion, loud noise, or touch. He had difficulty getting up from bed and performing routine activities for one month. Investigations regarding the autoimmune/thyroid/malignancy profiles were negative. The brain and spine MRI screen and chest, abdomen and pelvis CT were negative for malignancy. The paraneoplastic GAD antibody was positive. He was treated with baclofen, diazepam, clopidogrel, ropark, syndopa, escitalopram and five procedures of TPE, followed by an injection of methylprednisolone. The patient was able to arise from bed without the support and reported a 30 per cent improvement in imbalance, 60 % in blurred vision and 50 % in stiffness at the one-month follow-up verification (June 2021). He was able to walk independently with a slight dragging of the leg. The patient continued on oral medication and reported an 80% improvement in symptoms with medication in two follow-ups in December 2021 and March 2022. In November 2022, the patient presented with increased trunk muscle stiffness and difficulty in speech and breathing. His blood and CSF GAD antibodies were strongly positive. The patient was treated with MP (5 doses), followed by five TPE procedures. The patient reported a decrease in stiffness and frequency and was administered rituximab and discharged.
DiscussionThe TPE may have a role as adjuvant therapy in SPS patients along with other therapies. All patients in our study showed some degree of improvement following initial treatment. Pagano et al.1 and Albahra et al.7 documented variable improvement in SPS patients using TPE as a supportive therapy. A literature review of the Pubmed database (from 1988 to 2021) with keywords “therapeutic plasma exchange (TPE) in Stiff person syndrome (SPS)”, “role of plasma exchange in SPSS”, “plasmapheresis and SPSS treatment” and “stiff person syndrome treatment” yielded studies included in Table 2, which had utilized TPE as a therapeutic modality in SPS.
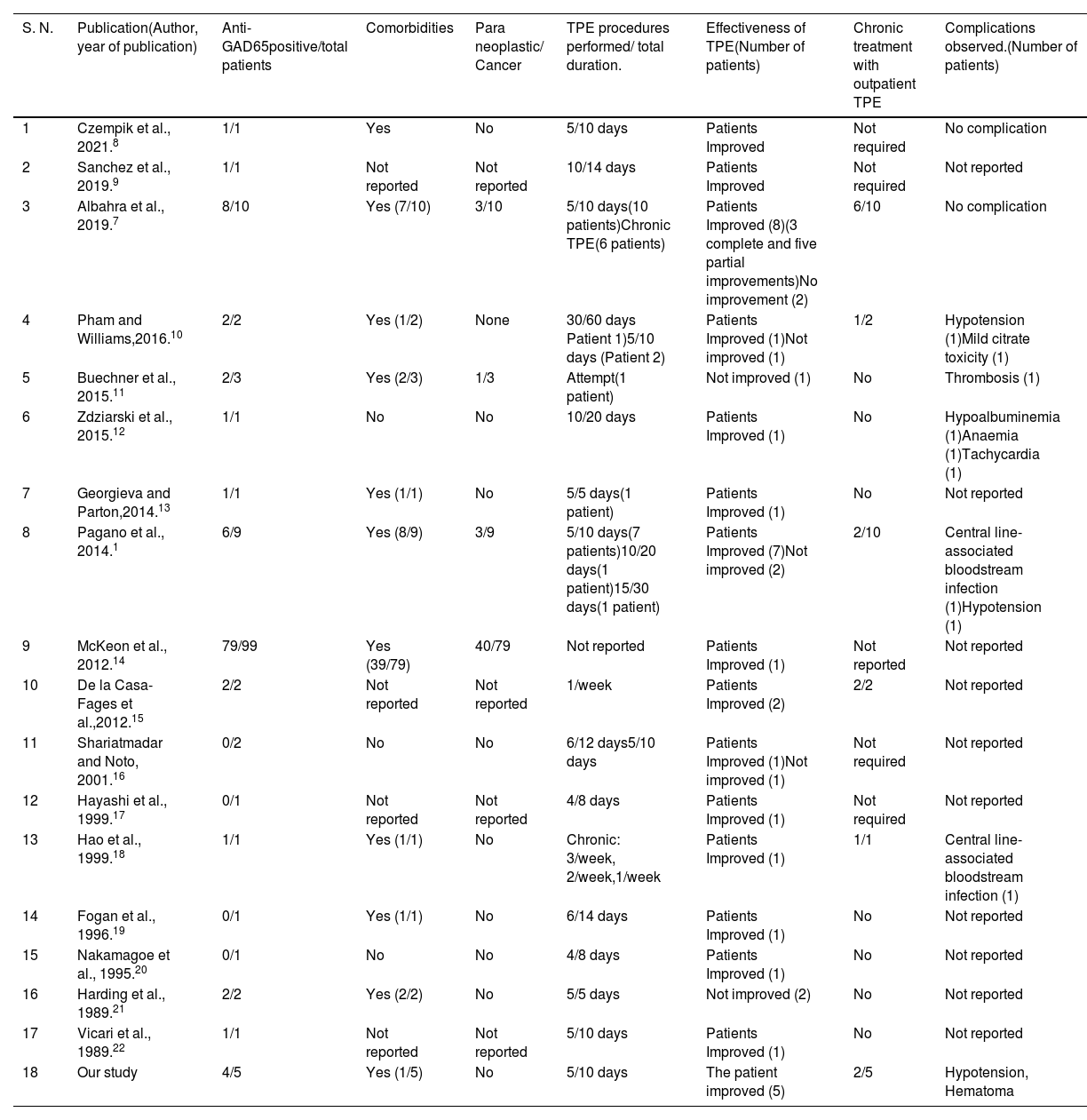
Previous studies reporting TPE as a therapeutic modality for SPS patients.
| S. N. | Publication(Author, year of publication) | Anti-GAD65positive/total patients | Comorbidities | Para neoplastic/ Cancer | TPE procedures performed/ total duration. | Effectiveness of TPE(Number of patients) | Chronic treatment with outpatient TPE | Complications observed.(Number of patients) |
|---|---|---|---|---|---|---|---|---|
| 1 | Czempik et al., 2021.8 | 1/1 | Yes | No | 5/10 days | Patients Improved | Not required | No complication |
| 2 | Sanchez et al., 2019.9 | 1/1 | Not reported | Not reported | 10/14 days | Patients Improved | Not required | Not reported |
| 3 | Albahra et al., 2019.7 | 8/10 | Yes (7/10) | 3/10 | 5/10 days(10 patients)Chronic TPE(6 patients) | Patients Improved (8)(3 complete and five partial improvements)No improvement (2) | 6/10 | No complication |
| 4 | Pham and Williams,2016.10 | 2/2 | Yes (1/2) | None | 30/60 days Patient 1)5/10 days (Patient 2) | Patients Improved (1)Not improved (1) | 1/2 | Hypotension (1)Mild citrate toxicity (1) |
| 5 | Buechner et al., 2015.11 | 2/3 | Yes (2/3) | 1/3 | Attempt(1 patient) | Not improved (1) | No | Thrombosis (1) |
| 6 | Zdziarski et al., 2015.12 | 1/1 | No | No | 10/20 days | Patients Improved (1) | No | Hypoalbuminemia (1)Anaemia (1)Tachycardia (1) |
| 7 | Georgieva and Parton,2014.13 | 1/1 | Yes (1/1) | No | 5/5 days(1 patient) | Patients Improved (1) | No | Not reported |
| 8 | Pagano et al., 2014.1 | 6/9 | Yes (8/9) | 3/9 | 5/10 days(7 patients)10/20 days(1 patient)15/30 days(1 patient) | Patients Improved (7)Not improved (2) | 2/10 | Central line-associated bloodstream infection (1)Hypotension (1) |
| 9 | McKeon et al., 2012.14 | 79/99 | Yes (39/79) | 40/79 | Not reported | Patients Improved (1) | Not reported | Not reported |
| 10 | De la Casa-Fages et al.,2012.15 | 2/2 | Not reported | Not reported | 1/week | Patients Improved (2) | 2/2 | Not reported |
| 11 | Shariatmadar and Noto, 2001.16 | 0/2 | No | No | 6/12 days5/10 days | Patients Improved (1)Not improved (1) | Not required | Not reported |
| 12 | Hayashi et al., 1999.17 | 0/1 | Not reported | Not reported | 4/8 days | Patients Improved (1) | Not required | Not reported |
| 13 | Hao et al., 1999.18 | 1/1 | Yes (1/1) | No | Chronic: 3/week, 2/week,1/week | Patients Improved (1) | 1/1 | Central line-associated bloodstream infection (1) |
| 14 | Fogan et al., 1996.19 | 0/1 | Yes (1/1) | No | 6/14 days | Patients Improved (1) | No | Not reported |
| 15 | Nakamagoe et al., 1995.20 | 0/1 | No | No | 4/8 days | Patients Improved (1) | No | Not reported |
| 16 | Harding et al., 1989.21 | 2/2 | Yes (2/2) | No | 5/5 days | Not improved (2) | No | Not reported |
| 17 | Vicari et al., 1989.22 | 1/1 | Not reported | Not reported | 5/10 days | Patients Improved (1) | No | Not reported |
| 18 | Our study | 4/5 | Yes (1/5) | No | 5/10 days | The patient improved (5) | 2/5 | Hypotension, Hematoma |
The mechanism of effectiveness of TPE in SPS is still not precise. One possible hypothesis is the removal of anti-GAD 65 antibodies and the immunomodulatory effect of TPE. This is supported by reports of cross-reactive anti-GAD antibodies post-West Nile virus infection in two patients, both of whom showed clinical improvement after TPE.7,23,24 The effect of TPE cannot be evaluated due to the simultaneous use of other medications required to relieve symptoms and immunomodulation.1,7 Other studies have reported no change in the level of anti-GAD antibodies after short and long-term TPE.8,15 Albahra et al. found no clear association between anti-GAD65 antibodies and disease activity.7 In our series, we have not quantitated the levels of anti-GAD antibodies.
The SPS is reported to be more prevalent in women than men3, but our series included four men and one woman, likely due to the inclusion of only those who underwent TPE. The TPE as an adjuvant treatment modality was used during the initial presentation at our institute in 4, and in 1 patient, it was used during relapse. Two patients (Cases 2 and 3) in our series received maintenance TPE procedures and showed improvement. The maintenance TPE in two SPS patients with severe symptoms showed improvement in spasm and stiffness previously.15 Anti-GAD antibodies were detected in 4 cases (80 %), similar to the frequency reported in the literature.25 The B cell-mediated autoimmune inflammation is the primary pathogenesis of SPS, resulting in the affection of different components of inhibitory GABAergic neuron synapses. More production of autoantibodies (anti-GAD antibodies) against antigens involved in the gamma-aminobutyric acid (GABA) synthesis leads to the dysfunction of major inhibitory pathways resulting in impaired truncal and axial muscle relaxation because of the hyperexcitability of the motor cortex.26 The primary pathological hallmark in classical SPS is the production of anti-GAD antibodies found in 70 to 80 % of classical SPS. Apart from classical SPS, anti-GAD antibodies have been associated with limbic encephalitis, autoimmune epilepsy, myoclonus, nystagmus and cerebellar ataxia. These disorders come under GAD antibody-spectrum disorders (GAD-SD).26
There is no randomized clinical trial to determine the efficacy of TPE in SPS patients due to the rarity of its occurrence and lack of available data. The ASFA 2023 guidelines reported more than 60 % of patients reporting some improvement after plasma exchange in SPS and placed TPE for SPS as a category III indication with a GRADE 2C recommendation. The TPE may be considered for patients not responding to conventional treatment, i.e., refractory ones. 6 Therapeutic plasma exchange effectively depletes the autoantibodies briefly when sufficient plasma volumes are removed. As our 4 out of 5 cases were anti –GAD positive, utilizing the TPE as a treatment modality was reasonable, along with adjuvant therapy. The IVIg, which has proven efficacy in randomized trials, was not offered to our patient population due to its high cost.6,27 The TPE has been reported to be more efficacious and cost-effective than IVIg in cost-constraint settings.28 The TPE may be reasonable in settings with no availability of IVIG or in a patient who does not respond to conventional therapy. Unlike previous studies, we did not observe any adverse events associated with the TPE procedure.1 There is no randomized clinical trial to determine the efficacy of TPE in SPS patients due to the rarity of its occurrence and lack of available data. The TPE can be a therapeutic option for SPS patients, particularly in resource constraint settings.
Our study has a few limitations. It is a retrospective study with only 5 cases. The cases were presented to us almost after 1 to 4 years of initial symptoms and received various treatments. The treatment effect of TPE cannot be assessed due to many confounding treatments. The clinical response prior to the TPE initiation and after each plasma exchange was not evaluated to determine SPS patient outcome. The levels of the anti-GAD65 antibodies were not quantified. However, the study demonstrates the effectiveness of TPE in treatment even in chronic cases and suggests utility in maintenance treatment, particularly in refractory cases.