Antiphospholipid syndrome (APS) is characterized by venous or arterial thrombosis, associated or not with pregnancy, in the presence of laboratory changes that show an autoimmune response through antiphospholipid antibodies.
Among the numerous antiphospholipid antibodies that can be titrated, it is important to measure anticardiolipin (aCL), lupus anticoagulant (LAC) and anti-Beta-2-glycoprotein-1 (B2-GPI). These have been shown to be more specific for confirming the diagnosis of APS in numerous series. 1
Antiphospholipid syndrome can be classified as primary or secondary. The primary form occurs in the absence of related or base diseases, being more common than the secondary, which is characterized by the association with a large spectrum of ilnesses. like systemic lupus erythematosus (SLE).
In both, thrombotic lesions are considered secondary vasculitis. Even in APS with no defined etiology, thrombosis is due to the known thrombophilic state, and cannot be labeled as primary vasculitis.2
In addition to the etiological criteria, APS can also be classified by the type of clinical manifestation into thrombotic (APS), obstetric (OAPS) or catastrophic antiphospholipid syndrome (CAS) or Asherson's syndrome. This classification allows stratifying patients in order to intensify therapy in those with greater morbidity.1
Both APS and CAS can present target organ lesions. In CAS, this injury is more pronounced and occurs in a short period of time. Some series reports of catastrophic antiphospholipid syndrome shows morbidity and mortality of up to 44%.
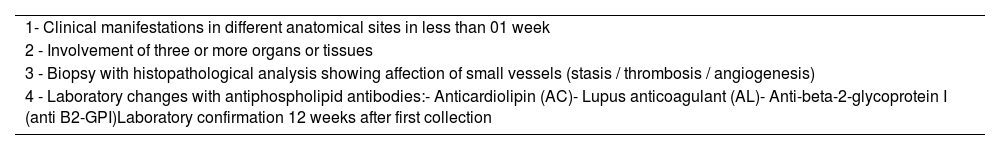
Some laboratory tests are common for the diagnosis of the 3 types of APS, but CAS requires additional diagnostic criteria listed below in Table 1. Under these conditions, CAS becomes rare, affecting less than 0.9% of patients with APS.1,3,4
Mandatory diagnostic criteria for CAS.
This report describes a rare case of CAS in a young woman who performed an accurate diagnosis, allowing specific treatment.
Case descriptionFemale, 24 years old and 10 months old, sought the emergency room of the University Municipal Hospital, complaining of pain in the right lower limb (RLL) due to multiple ulcerative lesions.
During the anamnesis, the patient reported:
- -
Use of rivaroxaban from 2013 to April 2018.
- -
Deep vein thrombosis (DVT) proximal of RLL in 2013 followed by pulmonary embolism (PE) and cardiorespiratory arrest.
- -
DVT of RLL in 2015.
- -
DVT in both lower limbs in 2016.
- -
DVT of RLL in 2017 with laboratory positivity for antiphospholipid antibodies. Dispite the laboratory diagnosis, she kept the rivaroxaban.
- -
PE in January 2018 with rivaroxaban.
- -
Passed vein cava filter in 2018
- -
Upper digestive hemorrhage in April 2018. Refers to substitute rivaroxaban for warfarin
- -
Did not perform medical evaluation in 2019
During the anamnesis, the patient denied alopecia, dyspnea, arthritic pain, fatigue, fever, hematuria, skin blemishes, dry eye and weight loss.
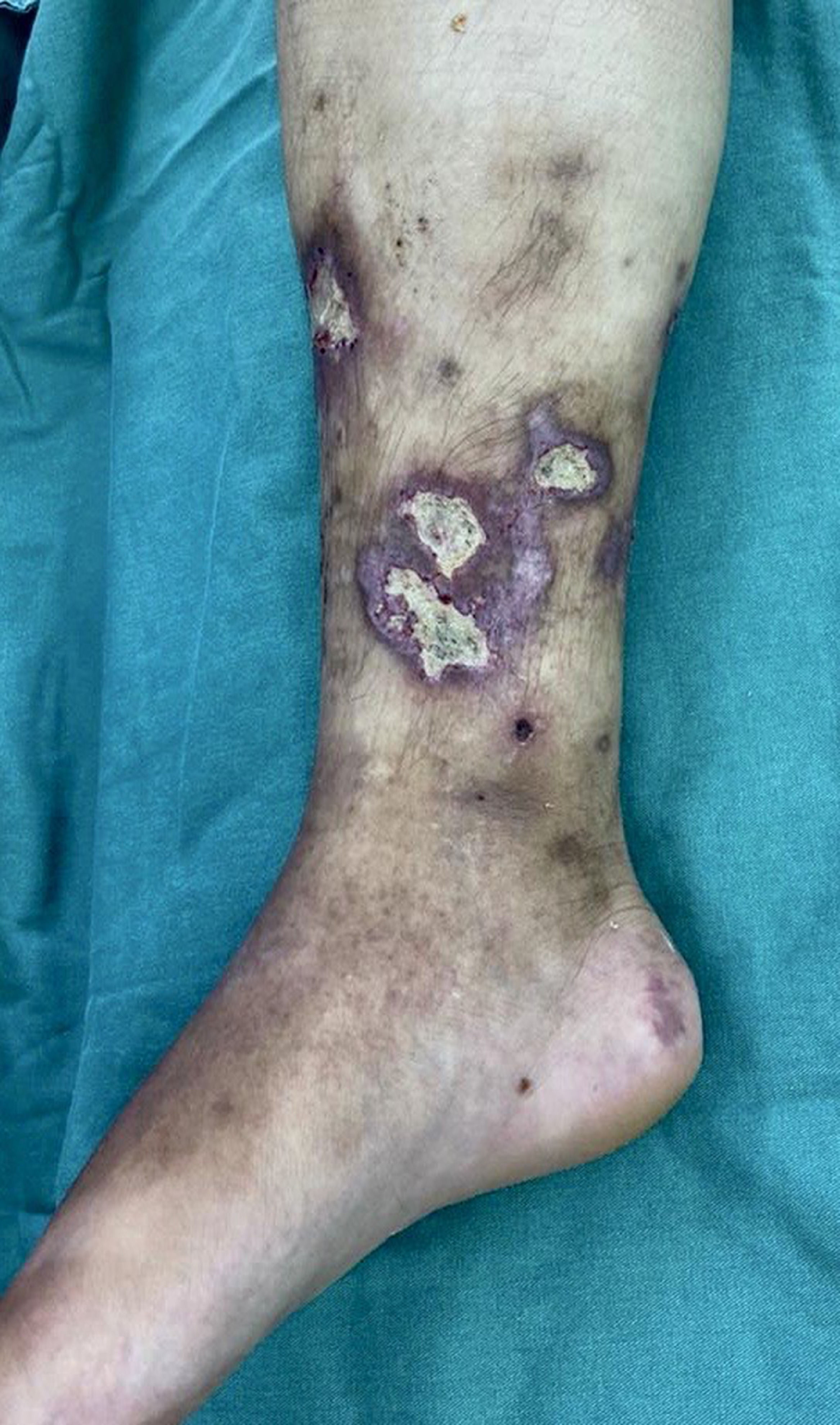
On physical examination in the emergency room, patient was active, eupneic, normotensive, absence of motor deficit in both upper and lower limbs, distal pulses present, edema of RLL ++ / 4, multiple ulcers infected in RLL with presence of necrotic tissue associated with fibrin and secretion as shown in Figure 1.
Complementary exams:
- -
Anticardiolipin (IGG antibodies): 79.3 GPL-U / ML (positive > 40 GPL-U / ML)
- -
Lupus anticoagulant (LAC):
Screening for abnormalities in phospholipid-dependent test
APTT (activated partial thromboplastin time) patient: 44.2 s patient / pool ratio of normal plasmas: 1.02 dRVVT (diluted Russel's viper venom test) patient: 39.7 s patient / pool ratio of normal plasmas: 1.28
Characterization of the inhibitor as phospholipid-dependent
Confirmatory APTT patient: 38.7 s patient / pool ratio of normal plasmas: 1.06
APTT ratio / confirmatory APTT ratio: 0.97 confirmatory dRVVT patient: 30.6 s patient / pool ratio of normal plasmas: 1.03 dRVVT ratio / confirmatory dRVVT ratio: 1.24
- -
B2-GPI - is not performed on our service.
- -
Anti-nuclear factor: reagent only for fine dotted standard core (others non-reagent)
- -
Rheumatoid factor (Waaler Rose): non-reagent
- -
63% prothrombin activity with 1,34 international normalized ratio (INR)
- -
Oxalacetic transaminase (GOT): 28 u / l (lower reference 40 U / L)
- -
Pyruvic transaminase (GPT): 17 u / l (lower reference 40 U / L)
- -
Blood-sedimentation speed: 100 mm (reference 2 to 10 mm)
- -
Hemoglobin: 10.6 g / dL (reference 12 to 16 g / dL)
- -
Transferrin saturation: 12% (reference 20% to 50%)
- -
Transferrin: 121 mg / dL (reference 200 to 360 mg / dL)
- -
Serum iron: 27 ug / dL (reference 37 to 145 ug / dL)
- -
Ferritin: 157 ug / dL (reference 10 to 291ug / dL)
- -
Protein electrophoresis: polyclonal hypergammaglobulinemia
- -
Leukocytes: 4,400 / uL (reference 3500 - 11000 uL)
- -
Platelets: 138000 / uL (reference 150000 - 450000 uL)
- -
Urea: 42mg / dL (reference 15-36 mg / dL)
- -
Creatinine: 0.6 mg / dL (reference 0.52-1.04 mg / dL)
- -
Urine I: without proteinuria and / or hematuria
- -
Color doppler ultrasonography with acute DVT in RLL.
- -
After headache. Magnetic resonance imaging of the skull with extensive thrombosis in the superior and transverse sagittal sinus.
In view of the information collected, it was imperative to perform a tissue biopsy in order to make the diagnosis of APS based on the diagnostic criteria already presented in Table 1.
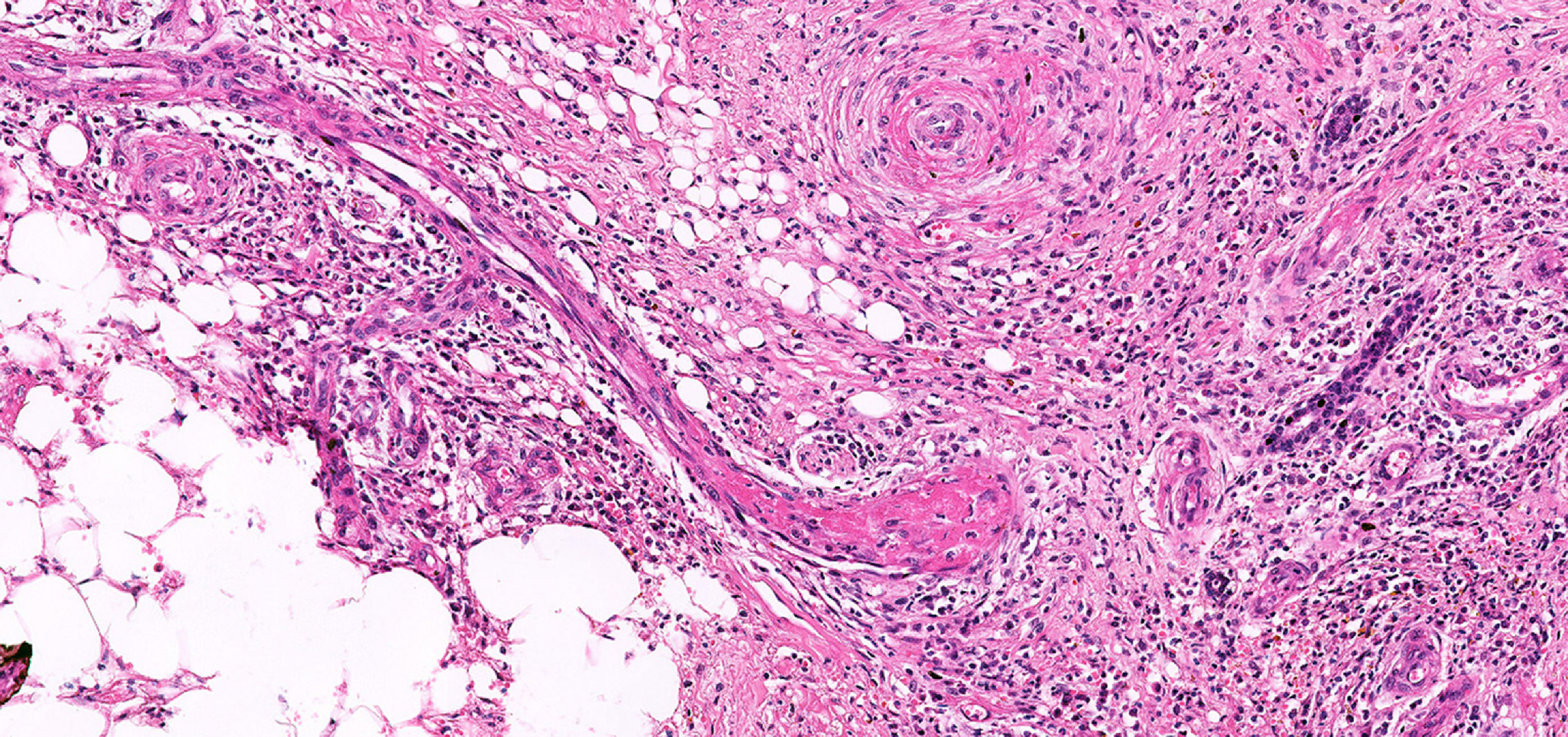
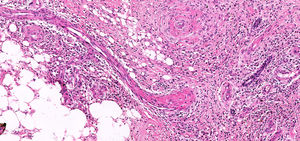
The RLL biopsy described “fibroleukocytic vasculitis in the dermis, atypia of newly formed capillaries and hypothesis of kaposiform lesion requiring immunohistochemistry”.
The immunohistochemical analysis concluded that it is a “chronic inflammatory process with capillary proliferation, thrombosis and absence of malignancy” (Figure 2).
DiscussionDuring the etiological investigation of CAS, numerous differential diagnoses such as thrombophilia and collagen diseases, should be investigated.
In these patients, it is common to find hemolytic anemia, idiopathic thrombocytopenic purpura, heparin-induced thrombocytopenia, Troussau syndrome, disseminated intravascular coagulopathy, sepsis, oncological disease and many others.4,5
Although some authors suggest that LAC research still requires standardization of technique and interpretation, we conclude that there is a presence of lupus anticoagulant.6
Despite the extensive possibility of differential diagnoses, Kaposi's sarcoma (SK) is not referred to in the literature as a differential diagnosis of CAS. In numerous publications such as Sapporo et al in 1999, Sydney et al in 2006 and Cervera et al in 2014, there is no mention of SK.1,7
Described since 1872, KS is an angioproliferative disease, associated with infection by human herpers virus 8 (HHV-8), classified into four aspects presented in Table 2.8
The DNA sequence of HHV-8 can be determined by the polymerase chain reaction. The presence of HVH-8 antigen is visualized by immunohistochemistry.9
Unlike KS, patients with APS must undergo intra-hospital anticoagulation with heparin and warfarin to the detriment of direct oral anticoagulants (DOACs), as it has been shown that in these patients the use of DOACS increases mortality and rate of new thrombotic events. INR between 2 and 3 is recommended as ideal. Pregnant women should receive low molecular weight heparin due to the possible teratogenic effects of warfarin.1,7
The reported patient started treatment with DOAC before the confirmed diagnosis of APS. After proper evaluation, she started using warfarin 5mg / day, reaching adequate INR and without signs of bleeding.
Due to the high recurrence of thrombotic events in CAS, anticoagulation is perennial.
The use of glucocorticoids in CAS can be performed at 0.5 to 1.0 g / day for 3 days. After the third day, oral prednisone is replaced with 1mg / kg / day.1
Our patient remains on 10 mg of prednisone per day.
There is no consensus in the literature on the use of intravenous immunoglobulins (IE). Some studies guide 400 mg / kg / day for 5 days in the acute phase.1
We do not use IE in this case.
Similarly to immunoglobulins, plasmapheresis also does not have randomized studies that allow use in patients with CAS.1
Some centers of excellence are using monoclonal antibodies anti CD20 (rituximab) or antiC5 (ecolizumab) in an experimental way.10
Refractory cases of CAS may also benefit from the association of statins, antiplatelet agents or immunomodulators such as chloroquine.
In addition to the rarity of the differential diagnosis with KS not found in the literature, the reported case of CAS demonstrates how morbidity and mortality are related to the correct and accurate diagnosis.