Autologous peripheral blood stem cell (PBSC) transplantation has become a standard treatment option for certain hematological malignancies. The collection of PBSCs for transplantation is a well-established process and the effectiveness can vary depending on the cell separator. We aimed to compare the effectivity of two devices, the Spectra Optia and the Amicus for autologous PBSC collection. We also evaluated the effect of the peripheral white blood cell (WBC) count on the CD34+ collection efficiency (CE2).
MethodsWe retrospectively evaluated 262 apheresis procedures performed in patients between 2015 and 2021 at the Apheresis Unit of our transplantation center. The PBSCs were collected by the Spectra Optia cell separator with continuous Mononuclear Collection (cMNC) (128 procedures) or the Amicus (MNC) (134 procedures). In addition to the apheresis parameters and product characteristics, we also evaluated the effect of the pre-apheresis peripheral WBC count on the CE2.
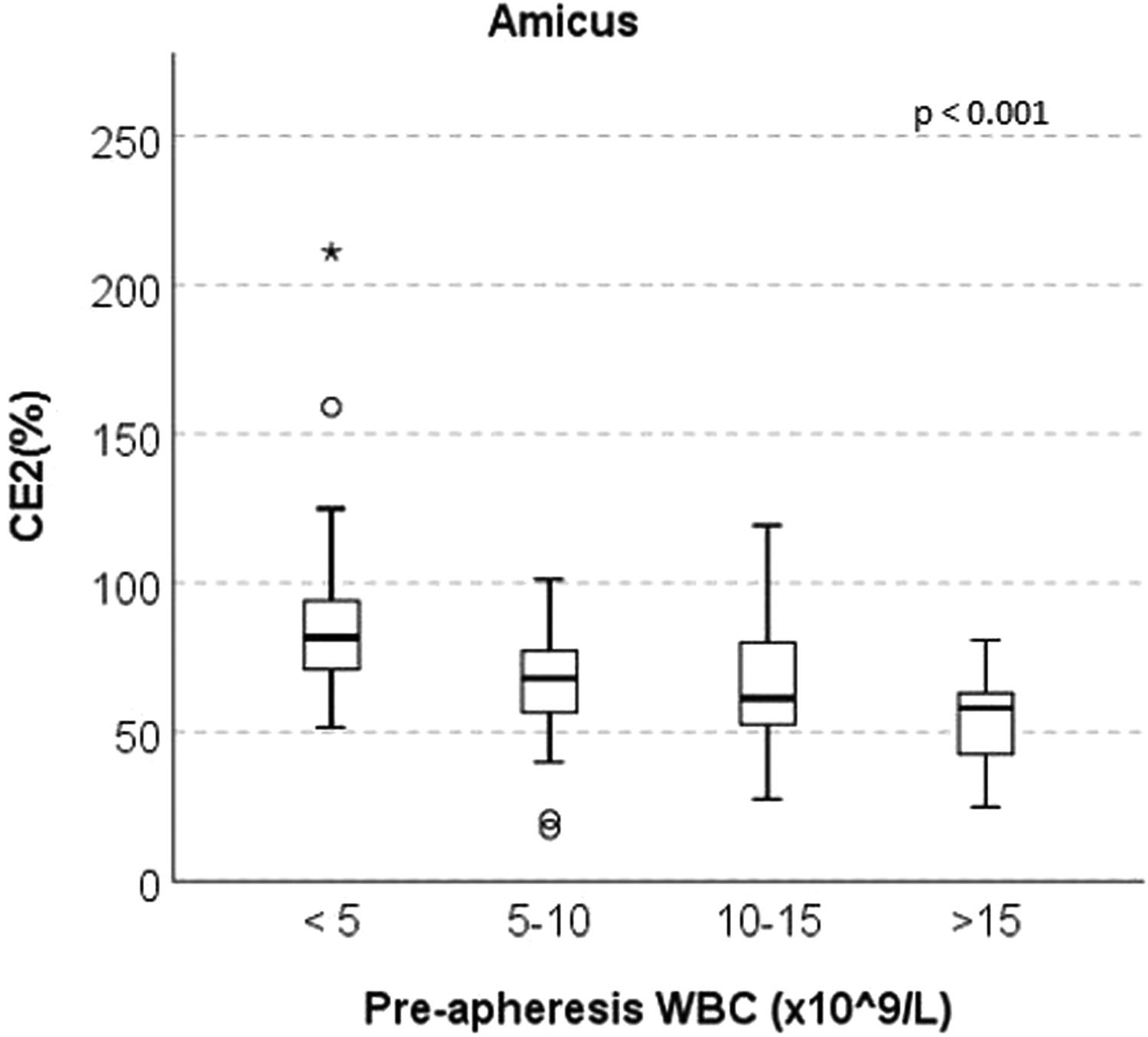
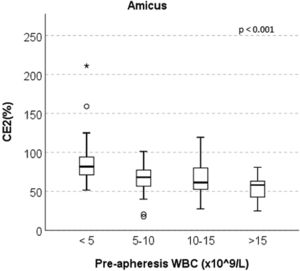
ResultsThere was no significant difference in the CD34+ CE2 between the Spectra Optia and Amicus devices (median 65.06% and 68.24%, respectively, p = 0.070). In the Amicus group, the CE2 ratio was found to be statistically significantly higher in patients with a pre-apheresis peripheral WBC count of 15 × 109/L (median 81.70%, 68.06%, 61.35% and 58.13%, respectively, p < 0.001).
ConclusionWhile both devices collected autologous PBSC effectively and safely, the Amicus provided a higher rate of CE2 at low pre-apheresis WBC counts. To our knowledge, this is the first study to evaluate the CE2 in autologous PBSC collection devices based on pre-apheresis WBC counts.
Autologous hematopoietic stem cell transplantation (HSCT) is an important treatment choice around the world for hematological malignancies, such as multiple myeloma and lymphoma. While it has a place in the first-line treatment in multiple myeloma, it also constitutes an important treatment approach in chemosensitive recurrent diffuse large B-cell lymphomas. Mobilized peripheral blood stem cell (PBSC) collection is preferred in 99% of autologous HSCT and efficient collection is essential for successful transplantation.1-4 The quality of the PBSC product, particularly the number and the viability of hematopoietic stem cells (CD34+ cells) is a crucial surrogate for successful engraftment. For three decades, there have been several cell separators used for PBSC collection worldwide 5-7 In the 2010s, the Spectra Optia device made it possible to collect cells with the continuous collection program called the continuous mononuclear cell collection (cMNC) protocol.8,9 The Amicus device, on the other hand, enables the collection of cells through cyclical cell harvests with the classical MNC protocol.10 Our department is a tertiary referral center for hematological diseases and Joint Accreditation Committee ISCT-Europe & EBMT (JACIE)-accredited for both autologous and allogeneic adult HSCT. Between 2015 and 2021, 836 HSCT (503 autologous and 333 allogeneic) have been performed. At our center, two apheresis devices [Spectra Optia (Terumo BCT, Lakewood, CO, USA) and Amicus (Fresenius-Kabi, Lake Zurich, IL)] are used for PBSC collection. The aim of our study is to compare the efficacy in collecting PBCSs with the Spectra Optia cMNC protocol, compared to the Amicus MNC protocol, focusing on procedure efficiencies and quality of products in autologous HSCT. In addition, this study aims to evaluate the effect of the pre-apheresis peripheral white blood cell (WBC) count on collection efficiency during autologous PBCS collection with current devices. While there are few reports in the literature in which the relatively new cMNC method in autologous PBSC collection is compared with the classical MNC method, we have not encountered any report that compared or even investigated the collection efficiency according to the WBC count before apheresis. In this sense, we aimed to determine which device would be appropriate to use according to the pre-apheresis WBC count and if the potential difference between the devices is shown, in terms of collection efficiency according to the pre-apheresis WBC count, this may be expanded in clinical practice.
MethodsPatients and methodsWe retrospectively analyzed 262 PBSC apheresis procedures performed in patients at the Apheresis Unit of Erciyes University between 2015 and 2021. There were 128 procedures in the Spectra Optia group and 134 procedures in the Amicus group. The study was approved by the Erciyes University Ethics Committee. The PBSC apheresis procedures were applied to patients who were 18 years of age or older and had signed a written consent form. Demographic and clinical features of the patients and mobilization regimens administered were recorded. In addition, apheresis parameters, such as procedure time, total processed volume (TPV), acid-citrate-dextrose (ACD) used, pre-apheresis peripheral blood CD34+ cell and WBC counts and product data, were evaluated. All laboratory tests were performed in the routinely used University Hospital Laboratory. Furthermore, all measurements of peripheral blood CD34+ cell count and CD34+ cell content of the product were performed by the FACS Calibur flow cytometer (Becton–Dickinson, Erembodegem, Belgium). For the collection of PBSCs, the Spectra Optia (Terumo BCT, Lakewood, CO, USA) and Amicus (Fresenius-Kabi, Lake Zurich, IL) were used at our center. For all apheresis procedures, the blood flow rate was 50–70 mL/min, and the whole blood to anticoagulant (ACD) ratio was 12:1. The PBSCs were collected according to our center's mobilization protocol using chemotherapy (cyclophosphamide/etoposide, cisplatin, cytarabine, prednisone (ESHAP)/ dexamethasone, cytarabine, cisplatin (DHAP)/ifosfamide, carboplatin, etoposide (ICE)) plus mobilization agents (granulocyte colony-stimulating factor (G-CSF), with or without plerixafor) or mobilization agents only. The peripheral blood CD34+ cell count was measured daily when the patient WBC count recovered to ≥2 × 109/L. Apheresis was started when the CD34+ cell count was ≥10/μL.11 The target CD34+ cell dose for one autologous HSCT was ≥3 × 106 cells/kg. The endpoint was a TPV of 2–4 times the patient estimated blood volume.12 All patients received continuous intravenous calcium gluconate (from 20 to 30 mL/h) to avoid citrate toxicity.
The collection efficiency of CD34+ cells (CE2) and percentages of pre-apheresis CD34+ cells were calculated by the following formulas:
CE2% = [(product CD34+ cells/µL × product volume (mL))/(pre-apheresis CD34+ cells/µL × total processed volume (mL)] × 100
Pre-apheresis CD34+ cells% = [pre-apheresis CD34+ cells (× 106/L)/pre-apheresis WBC (× 109/L)] × 100.13,14
Statistical analysisThe data were analyzed using the SPSS 26 (IBM SPSS Inc., Chicago, IL) software. The compatibility with normal distribution was examined with the Kolmogorov–Smirnov test. The independent samples t-test was used for the comparison of normally distributed parameters. The chi-square test was used to compare categorical variables. The Mann–Whitney U test was used for non-normally distributed parameters. Moreover, the Kruskal–Wallis test was used to compare WBC groups and the Bonferroni test was used for multiple comparisons. The accepted significance level was p < 0.05.
ResultsPatient characteristicsThe baseline characteristics of the patients are presented in Table 1. A total of 128 patients underwent mobilization with the Spectra Optia device and the other 134 patients, with the Amicus. While there were 78 males and 50 females in the Optia group, there were 80 males and 54 females in the Amicus group. Furthermore, 76 patients (59.4%) were mobilized with chemotherapy plus mobilization agents and 52 patients (40.6%), with mobilization agents only, in the Optia group, while 98 patients (73.1%) were mobilized with chemotherapy plus mobilization agents and 36 patients (26.9%), with mobilization agents only, in the Amicus group. The PBSC collection processes were performed in 72.5% of the patients from the central venous catheter and in 27.5% of the patients, from the peripheral venous access. All PBSC collection procedures were well tolerated and no serious adverse events were observed. In the Spectra Optia and Amicus groups, the most frequent hematologic disease was multiple myeloma (MM) (57.8% and 47%, respectively), followed by non-Hodgkin lymphoma (NHL) (28.1% and 30.6%, respectively) and Hodgkin lymphoma (HL) (9.4% and 19.4%, respectively). There were no significant differences in age, gender, weight, diagnosis or mobilization regimen between the groups.
Demographic and clinical characteristics of patients.
| Parameter | Spectra Optia (n = 128) | Amicus (n = 134) | P |
|---|---|---|---|
| Age (years)a | 54.5 (17–76) | 53 (19–81) | 0.689 |
| Gender (n, %) | 0.469 | ||
| Male | 78 (60.9) | 80 (59.7) | |
| Female | 50 (39.1) | 54 (40.3) | |
| Weight (kg)a | 77 (48–142) | 75 (50–110) | 0.211 |
| Diagnosis (n, %) | 0.413 | ||
| Multiple myeloma | 74 (57.8) | 63 (47) | |
| Non-Hodgkin lymphoma | 36 (28.1) | 41 (30.6) | |
| Hodgkin lymphoma | 12 (9.4) | 26 (19.4) | |
| Other | 6 (4.7) | 4 (3) | |
| Mobilization regimens (n, %) | 0.081 | ||
| Chemotherapy plus mobilization agents | 76 (59.4) | 98 (73.1) | |
| Mobilization agents only | 52 (40.6) | 36 (26.9) |
The apheresis parameters and collection outcomes are shown in Table 2. The procedure time was significantly larger in the Amicus group (mean 323.46 ± 64.96 min) than in the Spectra Optia group (mean 303.88 ± 65.54 min, p = 0.016). The processed blood volume was also higher in the Amicus group than in the Optia group (mean 16,388.56 ± 3393.22 vs. 15,016.09 ± 3813.37 mL, respectively, p = 0.002). There was no statistically significant difference in the ACD used, pre-apheresis CD34+ cell count and CE2 ratio between the groups. However, the pre-apheresis WBC count was statistically lower in the Amicus group than in the Optia group (median 6.61 (1.47–77) vs. 14.63 (1.84–85.90) × 109/L, respectively, p < 0.001). Likewise, the product volume and the product WBC count were lower in the Amicus group than in the Optia group (median 160 (64–400) vs. 218 (36–792) mL and median 181.60 (2.63–372.07) vs. 202.73 (25.79–656.48) × 109/L, p < 0.001 vs. p = 0.004, respectively). On the other hand, the product CD34+ cell count and product hematocrit (HTC) content was higher in the Amicus group than in the Optia group (median 4491 (552–65,343) vs. 2450 (333–50,131)/μL and median 8.15 (1–19) vs. 4.15 (1–15.7), respectively, p < 0.001). Furthermore, the product platelet (PLT) content was statistically significantly higher in the Spectra Optia group than in the Amicus group (median 1088.50 (32–4979) vs. 379 (24–1838) (× 109/L), respectively, p < 0.001)).
Apheresis parameters and collection outcomes.
| Parameter | Spectra Optia (n = 128) | Amicus (n = 134) | P |
|---|---|---|---|
| Procedure time (min) a | 303.88 ± 65.54 | 323.46 ± 64.96 | 0.016c |
| Processed blood volüme (mL)a | 15,016.09 ± 3813.37 | 16,388.56 ± 3393.22 | 0.002c |
| ACD used (mL) a | 1283.88 ± 331 | 1245.08 ± 289.86 | 0.313 |
| Pre-apheresis WBC (×109/L) b | 14.63 (1.84–85.90) | 6.61 (1.47–77) | <0.001c |
| Pre-apheresis CD34+ cells (/µL) b | 60 (5–973) | 65 (12–600) | 0.377 |
| Pre-apheresis CD34+ cells (%)b | 0.48 (0.02–22.89) | 1.05 (0.09–13.08) | <0.001c |
| Product volume (mL) b | 218 (36–792) | 160 (64–400) | <0.001c |
| Product WBC (×109/L) b | 202.73 (25.79–656.48) | 181.60 (2.63–372.07) | 0.004c |
| Product CD34+ cells (/μL) b | 2450 (333–50,131) | 4491 (552–65,343) | <0.001c |
| Product PLT (×109/L) b | 1088.50 (32–4979) | 379 (24–1838) | <0.001c |
| Product HTC (%)b | 4.15 (1–15.7) | 8.15 (1–19) | <0.001c |
| CE2 (%)b | 65.06 (30.15–343.21) | 68.24 (17.37–211.08) | 0.070 |
ACD: acid-citrate-dextrose; WBC: white blood cell; PLT: platelet; HTC, hematocrit; CE2: collection efficiency.
At the end of the study, four subgroups were formed for both devices based on the WBC counts after the median pre-apheresis WBC count was found to be statistically significantly lower in the Amicus group than in the Spectra Optia group (6.61 vs. 14.63 × 109/L), aiming to evaluate the CE2 ratios separately according to the pre-apheresis peripheral WBC counts of the groups. Interestingly, in the Amicus group, the CE2 was statistically significantly higher in patients with pre-apheresis peripheral WBC count of <5 × 109/L, compared to those with 5–10 × 109/L, 10–15 × 109/L and >15 × 109/L (median 81.70%, 68.06%, 61.35% and 58.13%, respectively, p < 0.001) (Figure 1). In the Spectra Optia group, no significant difference was observed between the groups in this regard.
DiscussionIn this study, our primary aim was to compare the Spectra Optia cMNC method with the Amicus MNC method in terms of collecting sufficient and high-quality products. Our secondary aim was to investigate whether collection efficiency is affected by the pre-apheresis WBC count. Both devices provided sufficient and safe collection. The results obtained regarding to the CE2 are consistent with the literature.15,16 In our study, the blood volume processed and, accordingly, the procedure time were higher in the Amicus group. On the other hand, the products are processed in cycles with the MNC protocol on the Amicus device, where it is expected for the procedure time to take longer since each cycle requires a certain break. In addition, the product volume was statistically lower in the Amicus group due to the technical characteristics of the device. Since the Amicus device processes high volumes of blood in each cycle, the product becomes more concentrated, thereby the product volume becomes lower. Also, it is predictable to obtain a higher amount of product PLT in the Spectra Optia group, while obtaining a higher amount of product HTC, in the Amicus group. That is because the Amicus device technically collects the buffy coat from deep regions during the collection of cells, that is, close to the area where the erythrocytes are located, therefore obtaining the product HTC in greater amounts. Since Spectra Optia technically collects the buffy coat near the surface, which is rich in PLTs, the product PLT content is also found to be high. While both groups exhibited a similar number of pre-apheresis peripheral blood CD34+ stem cells, the percentage of pre-apheresis CD34+ cells was higher in the Amicus group, which resulted from the low pre-apheresis WBC count in this group. Chung et al. reported no significant difference between the devices regarding pre-apheresis WBC counts.15 In our study, the pre-apheresis peripheral WBC count was statistically lower in the Amicus group, while the product CD34+ cell amount was higher. With the first results obtained in the study, the high pre-apheresis CD34+ cell percentage and high product CD34+ cell amount in the Amicus group, despite the lower pre-apheresis WBC count, led us to do the second part of the study, focusing on investigating the pre-apheresis WBC counts in four parts. Indeed, higher CE2 was found in those with a pre-apheresis WBC count <5 × 109/L in the Amicus group. This result suggested that the Amicus device can be preferred in patients with low pre-apheresis WBC counts. We believe that our study is the first study in which the Spectra Optia and Amicus devices are evaluated in terms of CE2 based on different pre-apheresis WBC counts in autologous PBSC collection. In a few studies in the literature, the contribution of pre-apheresis WBC counts to successful mobilization has been mentioned by looking at the donor data in allogeneic HSCT.1718 However, there are no reported studies on the contribution of different pre-apheresis WBC counts to CE2 in the autologous stem cell transplantation (auto-SCT). Chung et al. conducted the study in which Spectra Optia and Amicus devices were compared in collecting autologous PBSC, reporting no correlation between the pre-apheresis WBC count and product CD34+ cell count.15 The correlation between the WBC count and CE2 was not investigated in the study. In our study, the Amicus provided a higher rate of CE2 at low pre-apheresis WBC counts.
There are some limitations to our study. The first limitation might be the use of different mobilization regimens. Although it was not statistically significant, it was determined that the chemotherapy-supported mobilization agent was used more in the Amicus group. While there was no significant difference between the groups in terms of diseases, the difference in mobilization regimens may be due to the diversity in routine practices. For example, despite the fact that the autologous HSCT is the first option in transplant-eligible MM patients, there is still no consensus on the PBSC mobilization regimen.19,20 For this reason, only the mobilization agent was used in some MM patients and the chemotherapy-supported mobilization agent was used in others. On the other hand, in chemosensitive relapse/refractory lymphomas, which is the other group of diseases in which autologous HSCT is most frequently performed, chemotherapy-supported mobilization regimens are mostly preferred all over the world.21,22 In our study, such regimens were also used. The second limitation is the random selection of the devices used. As the Spectra Optia has wider indications for use (such as granulocyte/platelet apheresis and plasma exchange), when this device is occupied, the Amicus was preferred for the mobilization process.
In conclusion, our results demonstrated that autologous PBSC collection efficiencies were comparable between the two devices and that the Amicus provided a higher rate of CE2 at low pre-apheresis WBC counts. To our knowledge, this is the first study to evaluate the CE2 in autologous PBSC collection devices based on different pre-apheresis WBC counts. We think that our results will contribute to the literature in the field of autologous PBSC collection.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The author would like to acknowledge the assistance of the apheresis unit personnel.