Autologous stem cell transplantation is a treatment modality for several diseases. Prediction of successful mobilization may be useful to optimize hematopoietic stem cell collection.
Study design and methodsThis was a retrospective study with data from transplantation candidates between September 2015 and December 2021 being analyzed. The medical record of each patient was reviewed to mine mobilization information. The laboratory data analyzed were CD34+ cell enumeration and pre-collection peripheral blood cell count. The primary outcome, good mobilization, was defined as a CD34+ cell count ≥20/μL.
ResultsThis study included 807 patients. Increased patient weight, low mean corpuscular volume, high nucleated red blood cells, peripheral blood mononuclear cell and immature granulocyte counts were significantly associated with good mobilization. In addition, patients diagnosed with multiple myeloma were two times more likely to be good mobilizers than patients with lymphoma. The model was applied to a validation set to identify patients who underwent apheresis (CD34+ cell count ≥10 µL), resulting in a sensitivity of 69 %, a specificity of 95 %, positive predictive value of 98 %, and a negative predictive value of 50 %.
ConclusionSuccess in mobilization was greater in patients who underwent the first mobilization cycle and who had a diagnosis of multiple myeloma. Furthermore, higher body weight, and nucleated red blood cells, immature granulocytes and mononuclear cell counts, as well as low mean corpuscular volumes, were associated with successful mobilization.
Hematopoietic stem cell transplantation (HSCT) has been successfully used as a treatment for a wide variety of both hematological and non-hematological diseases.1–8 Cryopreservation has been routinely employed to maintain the viability and proliferative capacity of peripheral blood stem cells (PBSC) prior to autologous stem cell transplantation (ASCT).9–11 This therapeutic strategy has been shown to be safe and is associated with a low occurrence of significant side effects related to infusion and graft failure.
The most common strategy for PBSC mobilization consists of administering granulocyte colony stimulating factor (G-CSF) with or without chemotherapy to stimulate cell migration to the peripheral blood.2,12,13 There are several mobilization protocols, however, despite being a well-established procedure, prediction of successful mobilization is challenging. Several factors have been associated with the outcomes of mobilization, such as age, diagnosis, and previous chemotherapy or radiotherapy. Insufficient mobilization is a major impediment for performing ASCT with poor mobilizers having delayed neutrophil and platelet recovery even with similar infused CD34+ cell doses.3,5,14,15
The enumeration of CD34+ cells is considered the gold standard to define the best time to perform the collection of hematopoietic stem cells (HSC) by apheresis.6,13,14,16–18 Pre-apheresis CD34+ cell enumeration is used to guide the procedure with the result being directly related to the success of mobilization.14,16 However, CD34+ cell enumeration is an expensive and time-consuming procedure that requires trained staff and highly-specialized laboratory facilities.6–8,14,16,17,19 Thus, the number of laboratories that perform CD34+ enumeration is limited, especially in developing countries. Some transplant institutions outsource this procedure, which leads to operational and logistical difficulties for collection.
To date, there is no model to successfully predict mobilization, and surrogate markers of CD34+ enumeration are limited. Sysmex has developed a hematological parameter that identifies a population of immature myeloid peripheral blood cells, called “hematopoietic progenitor cells” (HPC), that is based on size, density and resistance to lysis.2,6,16,18,20 However, this is not an available feature of blood count analyzers in some countries, including Brazil. The aim of this study was to identify characteristics that might influence mobilization efficacy and to create a model for the prediction of stem cell mobilization in ASCT candidates. This study analyzed factors associated with successful mobilization and, separately, factors associated with inadequate mobilization.
Materials and methodsStudy design, patients and settingThis was a retrospective cross-sectional study involving individuals with hematological diseases, germ-cell tumors and solid tumors referred for ASCT at five transplant centers in the state of Minas Gerais, Brazil. Laboratory procedures were conducted at a single facility between September 2015 and December 2021 (Cetebio – Fundação Hemominas). All patients who had a PBSC collection attempt for ASTC in this timeframe were eligible for inclusion.
Exclusion criteria were: (1) quantification of CD34+ cells performed in a sample collected on the day before the collection attempt, and (2) donors undergoing collection for allogeneic transplantation.
Ethical approval, including a waiver of consent, was granted by the Institutional Review Board of “Fundação Hemominas” (CAAE: 23343019.2.0000.5118). The study was conducted in accordance with the Declaration of Helsinki.
OutcomesSuccessful mobilization (good mobilizers) was defined as pre-apheresis CD34+ cell quantification equal to or greater than 20 viable CD34+ cells/µL. The first collection attempt of each mobilization cycle was considered in the analysis of successful mobilization. The secondary outcome, poor mobilization, was defined as pre-apheresis CD34+ cell quantification less than 10 viable CD34+ cells/µL. Only the first day of the first mobilization cycle was considered in the analysis of the secondary outcome to prevent confounding bias, as poor mobilizers have higher odds of poor mobilization in subsequent mobilization attempts.
Clinical dataMobilization was achieved using G-CSF, associated or not with chemotherapy, administered subcutaneously according to the protocol established by each transplant center. Plerixafor was used in a small portion of patients who failed to reach the desired pre-apheresis CD34+ cell count. Pre-apheresis CD34+ cell count threshold for starting apheresis was ≥10 cells/µL.
Participants’ clinical data were obtained using a form completed by the transplant centers when requesting laboratory testing of samples. The following variables were collected: biological sex, diagnosis, age, weight, transplant center, number of mobilization cycles, type of mobilization, number of different prior chemotherapy regimens, prior radiotherapy, and pre-apheresis CD34+ and blood cell counts. For the descriptive analysis, a composite outcome was created for other plasma cell diseases including the diagnoses of amyloidosis, plasma cell leukemia, and POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, skin changes) syndrome.
Laboratory dataThe pre-apheresis CD34+ cell quantification was preferably performed with a peripheral blood sample collected between days 4.5 and 5.5 after the administration of G-CSF or during hematological recovery after chemotherapy and the administration of G-CSF.
Enumeration of CD34+ cells was achieved using a FACScalibur flow cytometer (Becton Dickinson, Palo Alto, CA, USA) and the International Society of Hemotherapy and Graft Engineering protocol (ISHAGE) on a dual platform (before September 2016)21 or single platform (after September 2016)22. The pre-apheresis peripheral blood cell count and CD34+ enumeration were performed using a Sysmex XN-1000 AS-01 automated cell counter (Roche, Basel, Switzerland).
Statistical analysisThe primary and secondary outcomes evaluated in this study were successful mobilization and poor mobilization, respectively. Laboratory data were not included in the analyses of the secondary outcome because the study aimed to evaluate the variables associated with poor mobilization at baseline.
Continuous variables are reported as medians (interquartile range) or means ± standard deviation (SD) depending on the distribution (normal distribution of continuous variables was verified by the Kolmogorov-Smirnov test). Categorical variables are summarized as frequencies and percentages of the total. For the descriptive analysis of patients with multiple collections, only the first collection was used. The association of continuous variables with the outcomes was examined using unpaired t-tests, except for variables with non-normal distributions, in which case the Mann–Whitney U test was used. Bivariable associations between categorical variables were evaluated using the two-tailed chi-square or Fisher's exact test.
The study population was randomly divided into derivation (80 %) and validation (20 %) datasets. Model derivation was performed using binary logistic regression to determine the independent effect of each covariate on good mobilization. The initial multivariable model included all covariates associated with each outcome (p-value <0.20) in the bivariable analysis. The covariates were removed from the model by backward elimination with the final models including only those covariates that were statistically significant with a p-value <0.01. Validity of the predictors was estimated by applying the final model to the validation dataset. Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and accuracy were assessed for the final model performance. Binary logistic regression was used to determine the independent effect of each covariate on mobilization.
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software version 21.0 (SPSS Inc.; Chicago, IL, USA).
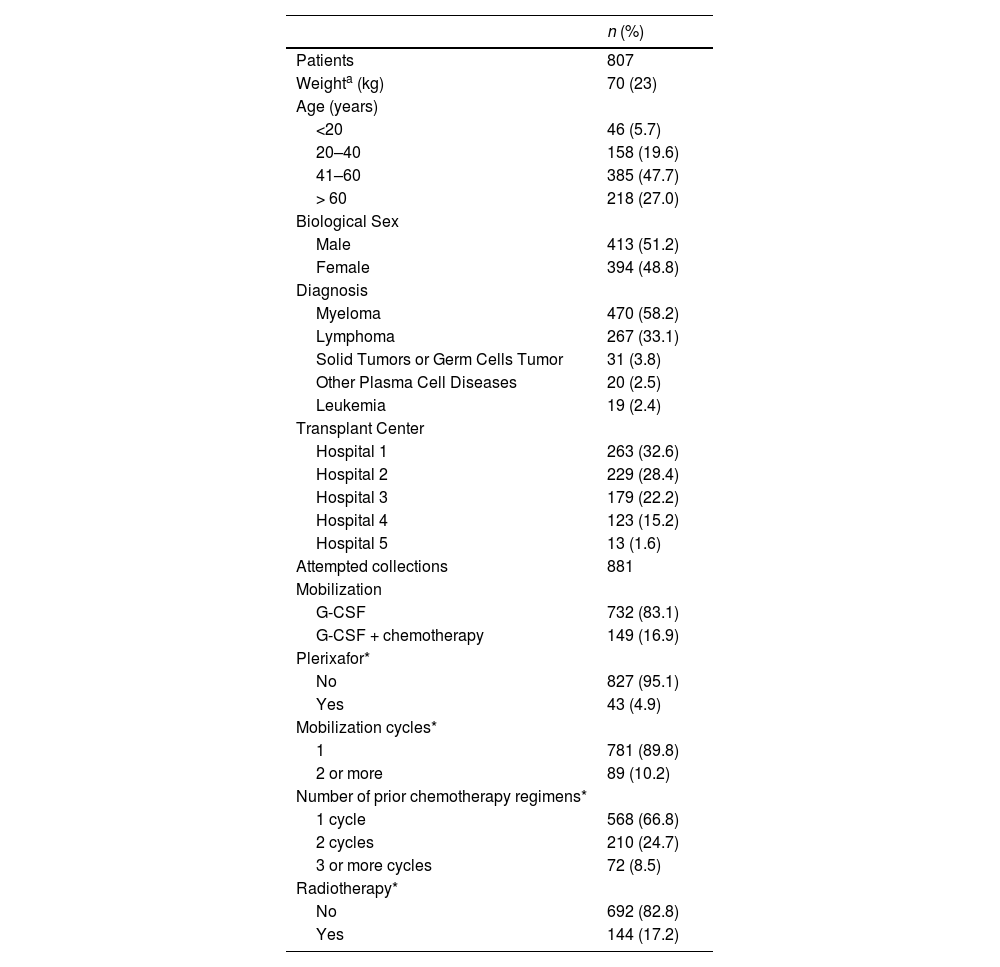
ResultsParticipant's characteristicsA total of 807 patients (51.2 % male; mean: 49.1 ± 15.9 years; range: 1–74 years) referred for ASCT were included. Myeloma was the most common diagnosis (58.2 %) followed by lymphoma (Table 1). Of the 807 patients, 74 had failed previous mobilization attempts, totaling 881 mobilization cycles and collection attempts in the study population.
Characteristics of candidates for autologous hematopoietic progenitor cell transplantation.
| n (%) | |
|---|---|
| Patients | 807 |
| Weighta (kg) | 70 (23) |
| Age (years) | |
| <20 | 46 (5.7) |
| 20–40 | 158 (19.6) |
| 41–60 | 385 (47.7) |
| > 60 | 218 (27.0) |
| Biological Sex | |
| Male | 413 (51.2) |
| Female | 394 (48.8) |
| Diagnosis | |
| Myeloma | 470 (58.2) |
| Lymphoma | 267 (33.1) |
| Solid Tumors or Germ Cells Tumor | 31 (3.8) |
| Other Plasma Cell Diseases | 20 (2.5) |
| Leukemia | 19 (2.4) |
| Transplant Center | |
| Hospital 1 | 263 (32.6) |
| Hospital 2 | 229 (28.4) |
| Hospital 3 | 179 (22.2) |
| Hospital 4 | 123 (15.2) |
| Hospital 5 | 13 (1.6) |
| Attempted collections | 881 |
| Mobilization | |
| G-CSF | 732 (83.1) |
| G-CSF + chemotherapy | 149 (16.9) |
| Plerixafor* | |
| No | 827 (95.1) |
| Yes | 43 (4.9) |
| Mobilization cycles* | |
| 1 | 781 (89.8) |
| 2 or more | 89 (10.2) |
| Number of prior chemotherapy regimens* | |
| 1 cycle | 568 (66.8) |
| 2 cycles | 210 (24.7) |
| 3 or more cycles | 72 (8.5) |
| Radiotherapy* | |
| No | 692 (82.8) |
| Yes | 144 (17.2) |
The most common peripheral blood stem cell mobilization regimen used G-CSF without chemotherapy. Plerixafor was used to increase the amount of circulating CD34+ cells in 43 (4.9 %) participants. Most mobilization attempts were preceded by one chemotherapy regimen treatment cycle, and 17 % were preceded by radiotherapy.
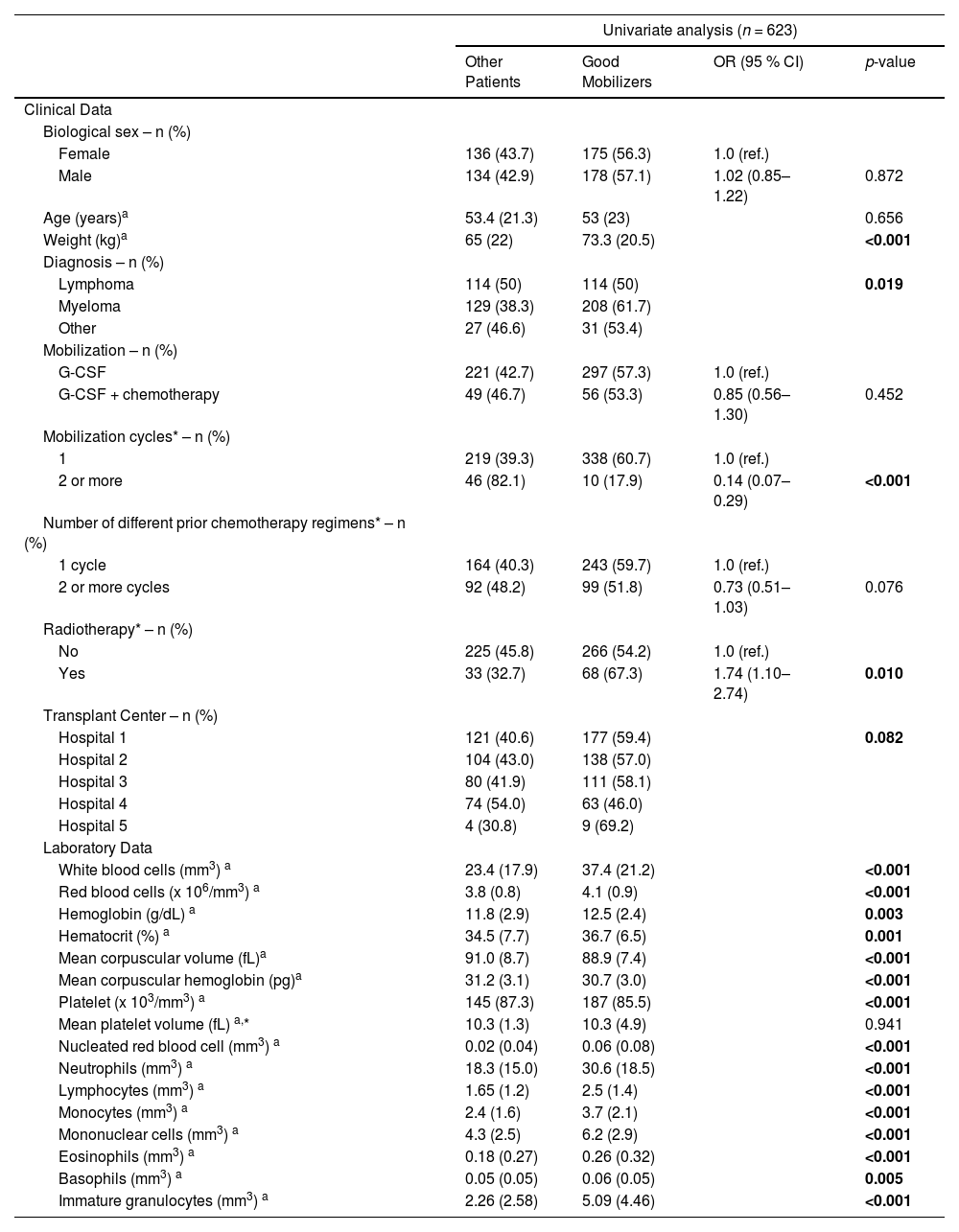
Association of clinical and laboratory characteristics with good mobilization: derivation of the modelGood mobilizers had significantly higher body weight when compared with those participants with a pre-apheresis CD34+ cell count <20 µL (Table 2). Additionally, most good mobilizers had multiple myeloma and were in the first cycle of mobilization. Good mobilizers had significantly higher red blood cell (RBC), platelet and white blood cell (WBC) counts and low mean corpuscular volume (MCV) and low mean corpuscular hemoglobin when compared to those with pre-apheresis CD34+ cell counts <20 µL.
Evaluation of the clinical and laboratory variables of successful mobilization in candidates for autologous hematopoietic progenitor cell transplantation – derivation cohort.
| Univariate analysis (n = 623) | ||||
|---|---|---|---|---|
| Other Patients | Good Mobilizers | OR (95 % CI) | p-value | |
| Clinical Data | ||||
| Biological sex – n (%) | ||||
| Female | 136 (43.7) | 175 (56.3) | 1.0 (ref.) | |
| Male | 134 (42.9) | 178 (57.1) | 1.02 (0.85–1.22) | 0.872 |
| Age (years)a | 53.4 (21.3) | 53 (23) | 0.656 | |
| Weight (kg)a | 65 (22) | 73.3 (20.5) | <0.001 | |
| Diagnosis – n (%) | ||||
| Lymphoma | 114 (50) | 114 (50) | 0.019 | |
| Myeloma | 129 (38.3) | 208 (61.7) | ||
| Other | 27 (46.6) | 31 (53.4) | ||
| Mobilization – n (%) | ||||
| G-CSF | 221 (42.7) | 297 (57.3) | 1.0 (ref.) | |
| G-CSF + chemotherapy | 49 (46.7) | 56 (53.3) | 0.85 (0.56–1.30) | 0.452 |
| Mobilization cycles* – n (%) | ||||
| 1 | 219 (39.3) | 338 (60.7) | 1.0 (ref.) | |
| 2 or more | 46 (82.1) | 10 (17.9) | 0.14 (0.07–0.29) | <0.001 |
| Number of different prior chemotherapy regimens* – n (%) | ||||
| 1 cycle | 164 (40.3) | 243 (59.7) | 1.0 (ref.) | |
| 2 or more cycles | 92 (48.2) | 99 (51.8) | 0.73 (0.51–1.03) | 0.076 |
| Radiotherapy* – n (%) | ||||
| No | 225 (45.8) | 266 (54.2) | 1.0 (ref.) | |
| Yes | 33 (32.7) | 68 (67.3) | 1.74 (1.10–2.74) | 0.010 |
| Transplant Center – n (%) | ||||
| Hospital 1 | 121 (40.6) | 177 (59.4) | 0.082 | |
| Hospital 2 | 104 (43.0) | 138 (57.0) | ||
| Hospital 3 | 80 (41.9) | 111 (58.1) | ||
| Hospital 4 | 74 (54.0) | 63 (46.0) | ||
| Hospital 5 | 4 (30.8) | 9 (69.2) | ||
| Laboratory Data | ||||
| White blood cells (mm3) a | 23.4 (17.9) | 37.4 (21.2) | <0.001 | |
| Red blood cells (x 106/mm3) a | 3.8 (0.8) | 4.1 (0.9) | <0.001 | |
| Hemoglobin (g/dL) a | 11.8 (2.9) | 12.5 (2.4) | 0.003 | |
| Hematocrit (%) a | 34.5 (7.7) | 36.7 (6.5) | 0.001 | |
| Mean corpuscular volume (fL)a | 91.0 (8.7) | 88.9 (7.4) | <0.001 | |
| Mean corpuscular hemoglobin (pg)a | 31.2 (3.1) | 30.7 (3.0) | <0.001 | |
| Platelet (x 103/mm3) a | 145 (87.3) | 187 (85.5) | <0.001 | |
| Mean platelet volume (fL) a,* | 10.3 (1.3) | 10.3 (4.9) | 0.941 | |
| Nucleated red blood cell (mm3) a | 0.02 (0.04) | 0.06 (0.08) | <0.001 | |
| Neutrophils (mm3) a | 18.3 (15.0) | 30.6 (18.5) | <0.001 | |
| Lymphocytes (mm3) a | 1.65 (1.2) | 2.5 (1.4) | <0.001 | |
| Monocytes (mm3) a | 2.4 (1.6) | 3.7 (2.1) | <0.001 | |
| Mononuclear cells (mm3) a | 4.3 (2.5) | 6.2 (2.9) | <0.001 | |
| Eosinophils (mm3) a | 0.18 (0.27) | 0.26 (0.32) | <0.001 | |
| Basophils (mm3) a | 0.05 (0.05) | 0.06 (0.05) | 0.005 | |
| Immature granulocytes (mm3) a | 2.26 (2.58) | 5.09 (4.46) | <0.001 | |
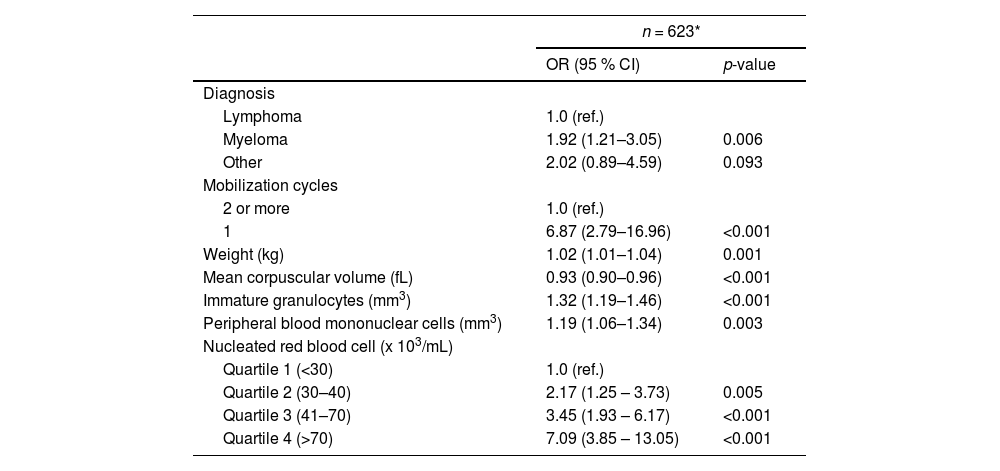
In the derivation cohort, diagnosis, number of mobilization cycles, higher body weight, low MCV, and increased peripheral blood mononuclear cells (PBMNC), nucleated red blood cell (nRBC) and immature granulocyte (IG) counts were significantly associated with successful mobilization in the final multivariable logistic regression model (Table 3 and Supplementary Table 1).
Multivariable model to examine characteristics associated with successful mobilization in candidates for autologous hematopoietic progenitor cell transplantation – derivation dataset.
| n = 623* | ||
|---|---|---|
| OR (95 % CI) | p-value | |
| Diagnosis | ||
| Lymphoma | 1.0 (ref.) | |
| Myeloma | 1.92 (1.21–3.05) | 0.006 |
| Other | 2.02 (0.89–4.59) | 0.093 |
| Mobilization cycles | ||
| 2 or more | 1.0 (ref.) | |
| 1 | 6.87 (2.79–16.96) | <0.001 |
| Weight (kg) | 1.02 (1.01–1.04) | 0.001 |
| Mean corpuscular volume (fL) | 0.93 (0.90–0.96) | <0.001 |
| Immature granulocytes (mm3) | 1.32 (1.19–1.46) | <0.001 |
| Peripheral blood mononuclear cells (mm3) | 1.19 (1.06–1.34) | 0.003 |
| Nucleated red blood cell (x 103/mL) | ||
| Quartile 1 (<30) | 1.0 (ref.) | |
| Quartile 2 (30–40) | 2.17 (1.25 – 3.73) | 0.005 |
| Quartile 3 (41–70) | 3.45 (1.93 – 6.17) | <0.001 |
| Quartile 4 (>70) | 7.09 (3.85 – 13.05) | <0.001 |
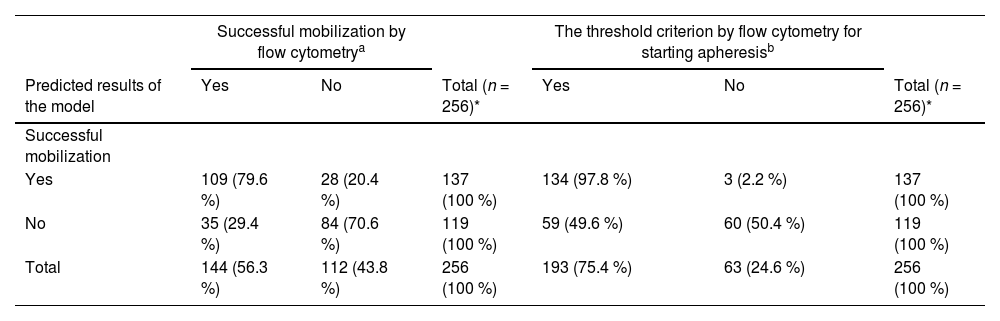
The final model showed an overall accuracy of 75 %, sensitivity of 76 % and specificity of 75 % for the validation set. The PPV was 80 %, and the NPV was 71 %. The model was also applied to the validation set to identify participants who underwent apheresis (viable CD34+ cell count ≥10 µL), resulting in a sensitivity of 69 %, a specificity of 95 %, a PPV of 98 %, a NPV of 50 % and an overall accuracy of 76 % (Table 4).
Validation of the model to identify mobilization status in candidates for autologous hematopoietic progenitor cell transplantation – validation dataset.
| Successful mobilization by flow cytometrya | The threshold criterion by flow cytometry for starting apheresisb | |||||
|---|---|---|---|---|---|---|
| Predicted results of the model | Yes | No | Total (n = 256)* | Yes | No | Total (n = 256)* |
| Successful mobilization | ||||||
| Yes | 109 (79.6 %) | 28 (20.4 %) | 137 (100 %) | 134 (97.8 %) | 3 (2.2 %) | 137 (100 %) |
| No | 35 (29.4 %) | 84 (70.6 %) | 119 (100 %) | 59 (49.6 %) | 60 (50.4 %) | 119 (100 %) |
| Total | 144 (56.3 %) | 112 (43.8 %) | 256 (100 %) | 193 (75.4 %) | 63 (24.6 %) | 256 (100 %) |
Seven hundred and eight one (89.8 %) patients were in the first collection attempt of the first mobilization cycle. Low body weight and number of prior chemotherapy regimens were statistically significant in respect to poor mobilization in the multivariable logistic regression model (Table 5).
Multivariable model to predict poor mobilization in candidates for autologous hematopoietic progenitor cell transplantation.
Although the pre-apheresis enumeration of CD34+ cells is known to be the gold standard to define the timing of HSC collection by apheresis, the results of the current study show that other clinical and laboratory data are associated with pre-apheresis CD34+ cell counts and can be useful during the collection process. Multiple myeloma diagnosis, only one mobilization cycle, and high nRBC, PBMNC and IG counts as well as low MCV were significantly associated with good mobilization. On the other hand, low body weight and two or more different chemotherapy regimens were significantly associated with poor mobilization.
G-CSF is a drug used to treat secondary neutropenia and acts to control hematopoiesis.12,15,23,24 According to the instructions provided by the manufacturer, G-CSF is a glycoprotein that regulates the production and release of functional neutrophils from the bone marrow. Furthermore, it induces secondary increases in circulating eosinophils and basophils. The IGs and nRBCs are precursor cells and we believe that the use of G-CSF could also stimulate the migration of these cells to the peripheral blood. The intense stimulation of cell production could generate smaller cells, which would explain the lower MCV in good mobilizers.
The size and internal complexity of HSCs are similar to those of monocytes and lymphocytes.25 Due to these characteristics, HSCs can be read as PBMNC in a automatic hematology counter, so an increase in the PBMNC count in good mobilizers is expected.
Initially, it was believed that the WBC count and the number of HSCs collected by apheresis were correlated. However, several studies have demonstrated the absence of any correlation between WBC and the enumeration of CD34+ cells in peripheral blood.7,13,14,16,17,26 The data of the present study corroborates previous studies showing an absence of an association between WBC and the pre-apheresis CD34+ cell count.
Several studies demonstrated a good correlation between the Sysmex HPC parameter and pre-apheresis CD34+ cell counts. However, reports also describe that this association may vary depending on the patient's disease and the mobilization regimen used.2,6,7,16–20 Despite the strong correlation found in the literature, the HPC parameter is not available on equipment sold in some countries, which makes its wide-spread use unfeasible. This study found other parameters associated with pre-apheresis CD34+ cell enumeration. These parameters could be used as an alternative to optimize HSC harvesting by apheresis.
Acquisition of new equipment involves complex logistics and high costs. Using data already available with current instrumentation is a way to optimize financial resources and improve the services offered to transplant centers. The results of this study are extremely relevant to laboratories and institutions that do not have a flow cytometer and rely on other institutions to define HSC collection by apheresis. Collection of HSC by apheresis and enumeration of pre-apheresis CD34+ cells are currently performed in different facilities separated by more than 40 km distance from our institution. The average time required to transport the sample, perform the CD34+ enumeration test, and release the result is 2.5 h. Our objective was to develop a model based on clinical and laboratory characteristics that predict successful mobilization to enhance PBSC collection in such institutions. The validation data show that the use of the model would rarely lead to starting an apheresis procedure in a patient with pre-apheresis CD34+ cell counts <10 µL. Although some good mobilizers are not identified by the model due to its low NPV, the only consequence for these patients would be to wait for the CD34+ count test by flow cytometry. The predictive accuracy of our model is also a step toward the development of accurate prognostic tests to identify individuals at risk of unsuccessful mobilization and to help select better therapeutic options.
The model may provide some benefits in the clinic. The first one is the optimization of the collection and the possibility of scheduling more than one collection per day. Starting a collection in the early morning would enable another collection in the afternoon, which would benefit patients waiting for an opportunity to undergo transplantation. In addition, it would allow CD34+ cell enumeration in the leukapheresis product on the same day in institutions that do not have night shifts. The enumeration of PBSC yield in apheresis procedures on the same day would bring several benefits to patients and transplant centers: (1) removal of the catheter on the same day, reducing the inconvenience caused and the risk of infections; (2) absence of the need to administer another dose of the mobilization regime preventively; (3) early release of a hospital bed that could be used for another patient.
The identification of characteristics associated with poor mobilization enables the adoption of additional clinical strategies for patients at high risk of unsuccessful mobilization. For example, if a patient is referred for ASCT and has already undergone several chemotherapy regimens, the transplant center could plan the mobilization with plerixafor. Other studies have identified other factors associated with poor mobilizers, such as age, prolonged chemotherapy and previous and extensive radiotherapy. Although researchers have tried to define characteristics associated with poor mobilization, as yet there is no consensus.2,3,19
Limitations of this study include its retrospective design and missing data on patients’ charts. Another limitation is the absence of patient height to calculate the body mass index (BMI); its use instead of weight would be more reliable. It is known that obesity can influence transplant outcomes and the best to calculate it would be through the BMI.27–29 Finally, there is a need to externally validate the prediction model in an independent population.
In conclusion, successful mobilization was more common in participants with higher weight, those undergoing their first mobilization cycle, and those with a diagnosis of multiple myeloma. Furthermore, high PBMNC, nRBC and IG counts as well as low MCV were associated with successful mobilization. A predictive model using these variables was established to identify successful mobilization. This model was validated in a subset of a study population and it identified participants who achieved successful apheresis with 76 % accuracy. These data can be used to help streamline and optimize the collection of HSC by apheresis.