Umbilical cord blood is an alternative source of hematopoietic progenitor cells for bone marrow transplantation; however, it is associated with a higher graft failure rate. The presence of a high rate of nucleated red blood cells (NRBCs) seems to be related to a greater capacity for engraftment, although is also associated with fetal distress conditions. We analyzed the correlation of the NRBC with quality parameters and its association with the utilization score of a cord blood unit.
Study design and methodData of 3346 units collected in a public cord blood bank from May 2010 to December 2017 were analyzed, retrospectively, to identify factors associated with an increased number of nucleated red blood cells and its correlation with the engraftment capacity measured through total nucleated cells (TNCs) and CD34 positive cells. We also evaluated the utilization score of these units and identified an NRBC cutoff associated with a higher score.
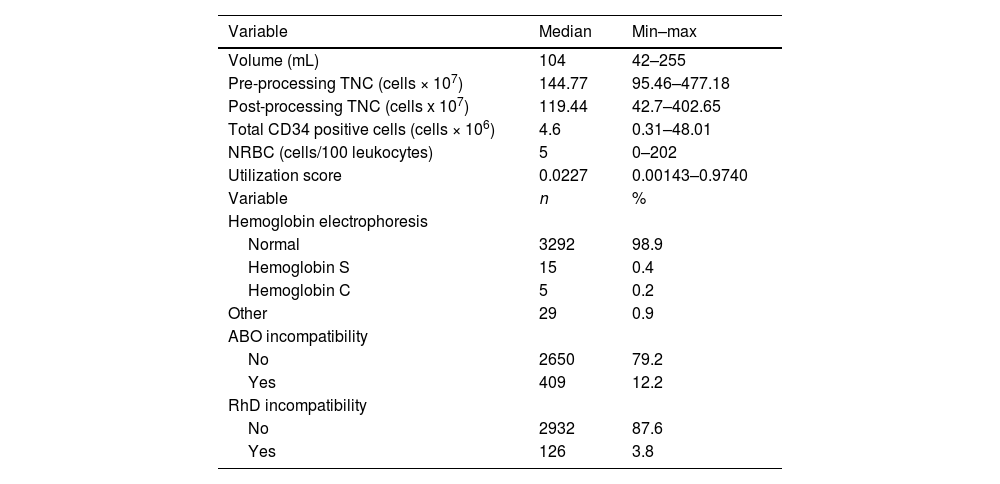
ResultsThe median volume collected was 104 mL (42–255), the pre-processing TNC count was 144.77 × 107 (95.46–477.18), the post-processing TNC count was 119.44 × 107 (42.7–477.18), the CD34 count was 4.67 × 106 (0.31–48.01), the NRBC count was 5 (0–202) and the utilization score was 0.0228 (0.00143–0.9740). The NRBC showed a correlation with the collected volume, TNC and CD34 positive cells and a higher utilization score and the receiver operating characteristic (ROC) curve analysis identified the five NRBC/100 leukocytes cutoff that correlates better with the probability of use. No association with pathological conditions and the NRBC rate was observed.
ConclusionsThe NRBC is a feasible parameter for the screening of the cord blood unit (CBU) and the minimum cutoff of five NRBC/100 leukocytes can be a strategy in conjunction with the TNC to identify better units for cord blood bank sustainability.
The first cord blood transplant was performed in 1989. Since then, the cord blood unit (CBU) has become one of main sources of hematopoietic progenitor cells (HPCs) in poorly represented groups in voluntary bone marrow donors.1,2 However, the higher rate of graft failure has resulted in several studies being undertaken to identify factors that are associated with better engraftment.1,3 The total nucleated cells (TNCs), amount of CD34 positive progenitor cells, cell viability and number of colony-forming units (CFUs) in the clonogenic assay are variables associated with the grafting potential of the cord blood unit (CBU) and their determination before the unit selection improves the outcome in the cord blood transplant.4–7
Meanwhile, haploidentical transplantation has been increasingly used, based on the improvement in strategies to reduce the alloreactivity between donors and receptors. This type of transplant has the advantages of faster availability of the donor and greater access to collect additional cellular products in situations of graft failure or relapsed disease. As a result, haploidentical transplants have reduced cord blood selection for transplants and cord blood banks are looking for strategies to optimize their inventory, striving to maintain units with higher probability of use and greater sustainability.8
Public banks established the criteria for units that should be cryopreserved based on the TNCs and CD34 positive cells and some studies have suggested applying scoring systems obtained by quality parameters of the CBU before storage.9,10 To implement these scores, it is necessary to perform tests by flow cytometry, which impact in costs; however, without new strategies, cord blood banks could evolve into bankruptcy.
On the other hand, new research is investigating cord blood cells as a source of stem cells for regenerative medicine, which seems to be a good alternative use of the CBU.11 Evaluations of better units for the bone marrow transplant (BMT) by a scoring system should be a method for the selection of inventory intended for research and for transplantation.
Thus, considering the economic impacts of the cord blood bank maintenance, the costs of performing all quality parameters and the necessity to identify the units to be used for research, this study retrospectively analyzed CBU data and considered the TNCs and CD34 correlation with other parameters, such as the collected volume and total nucleated red blood cells (NRBCs). This study evaluated if these parameters could be representative of the CBU proliferative potential and could be an easier way to better screen units for the bone marrow transplant. As the nucleated red blood cell can be associated with asphyxia or hemolytic disease, this study also verified its correlation with the ABO and RhD incompatibility and presence of variant hemoglobin, trying to exclude situations in which the NRBC is associated with a pathological condition with enhanced erythropoietin activity and does not represent the CBU proliferative condition.12–15
MethodsA retrospective study was performed, through data of cord blood units of a public cord blood bank in Sao Paulo, Brazil. The entire process of collection, processing and cryopreservation followed recommendations published in the Brazilian Ministry of Health Standards and International Quality Standards Guidelines.
After approval by the institutional ethics committee (CAAE: 41687914.2.0000.0071), the following data were collected:
- 1.
Post-processing total nucleated cells
This quantification was performed using the Pentra 120 DX Hematological Analyzer (Horiba Medical, Kyoto, Japan) and from 2012 using the XE2100 Analyzer (Sysmex Co. Kobe, Japan).
Total nucleated cells were determined by:
- 2.
CD34 positive cells
The amount of CD34 positive cells was measured on the Coulter Epics XL-MCL Flow Cytometer (Beckman Coulter, Bres, CA, USA), according to the International Society of Hemotherapy and Graft Engineering (ISHAGE) protocol, with a double platform.16
The total of CD34 positive cells of the product was calculated by:
Since 2015, a single platform based on the ISHAGE guidelines was established and total CD34 positive cells were determined by:17
- 3.
Nucleated red blood cells
Nucleated red blood cells (NRBCs) were determined manually through the analysis of the peripheral blood smear submitted to the May–Grunwald–Giemsa stain, modified by the Rosenfeld Fonio Method, was used to measure the rate of NRBCs/100 leukocytes.18 Since 2012, the NRBC count started to be measured automatically by the XE2100 equipment (Sysmex Co., Kobe, Japan). Units with counts above 30 NRBCs/100 leukocytes were subjected to confirmation by manual count of the peripheral blood smear.
- 4.
Utilization Score of the CBU
This score was calculated based on a previous study that determined the probability of the CBU selection for transplantation.10
In this study, units from four cord banks (two in France, one in Germany and one in the United States) were retrospectively analyzed based on total nucleated cells, CD34 cell count and HLA haplotype, divided into Caucasian and non-Caucasian groups, based on the National Marrow Donor Program (NMDP) database, to identify parameters associated with a higher probability of being transplanted.
A logistic regression model was performed to assess the level of influence of these three parameters on the probability of use. Parameters with the lowest value of the Akaike Information Criterion were selected to derive a formula that reflects the probability of use, defined as the utilization score index of the CBU.10
The utilization score was calculated by equation:
TCN cells × 108 and CD34 cells × 106
In this previous study, a receiver operating characteristic (ROC) analysis of this score determined maximal sensitivity and specificity at the score of 0.0308.10 The CBUs were divided into two groups: Group A with the score up to 0.0308 and Group B with the score over 0.0308.
- 5.
Newborn hemoglobin electrophoresis
All units were evaluated for the presence of variant hemoglobin using the electrophoresis technique in alkaline pH (cellulose acetate) and confirmed in an electrophoresis at an acid pH to identify hemoglobin S and C.19
- 6.
ABO and RhD incompatibility
ABO incompatibility was defined in situations in which the mother had blood group O and the newborn had any blood type other than group O (A, B or AB). The RhD incompatibility was defined as those situations in which the mother was RhD negative, with the presence of the anti-D antibody and the newborn had positive RhD typing. Both conditions could lead to newborn hemolytic disease.20,21
This study included all the CBUs collected from May 2010 to December 2017, which met the criteria for cryopreservation, consisting of a minimum initial count of 950 million cells pre-processing and units from mothers who signed informed consent for CBU donation. Related CBUs were excluded from this study.
StatisticsThe CBU data, such as TNCs, total CD34 positive cells, NRBC and utilization score, were expressed as median, minimum and maximum values. Normal distribution of variables was verified by the Kolmogorov–Smirnov test and by the graphical analysis. Qualitative variables were expressed in terms of absolute and relative frequencies (percentage). The Mann–Whitney test was used to compare these data between groups.
The intensity of the relationship between the variables was verified by Spearman's correlation coefficient. The linear regression model was complemented to estimate the rate of erythroblasts according to the TNCs, CD 34 positive cells, score rate and presence of variant hemoglobin and ABO or RhD incompatibility. The ROC curve was performed to identify sensitivities and specificities for different values of the NRBC for the utilization score.
The data were tabulated using the SPSS software for Windows version 22.0 (IBM). The test results were evaluated when the descriptive level (p-value) was less than 0.05.
ResultsIn this period, 6707 CBUs were collected, of which 3346 units met the acceptable criteria and were analyzed in this study.
The median of volume collected was 104 mL (42–255), the pre-processing TNC count was 144.77 × 107 (95.46–477.18), the postprocessing TNC, 9.44 × 107 (95.46–477.18), the CD34, 4.67 x 106 (0.31–48.01), the NRBC, 5 (0–202), and the utilization score was 0.0228 (0.00143–0.9740). Variant hemoglobin was identified in 1.5% of the CBUs (49 units) and ABO and RhD incompatibility were described in 12.2% (409) and 3.8% (126), respectively, as described in Table 1.
Demographic characteristics of cord blood units.
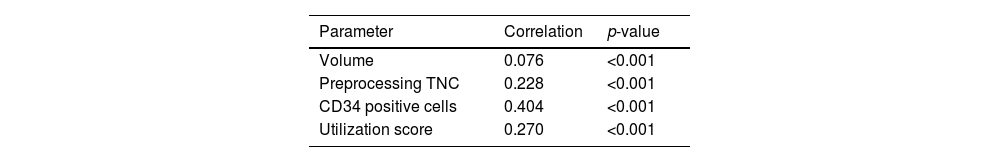
The NRBC showed a correlation with the collected volume, TNCs and CD34 positive cells and had a higher utilization score, as described in Table 2. The CBUs were divided into two groups according to the utilization score: Group A, with 2083 units (62.3%) and the score below 0.0308, and group B, with 1246 units (37.2%) and the score equal to, or over 0.0308. Considering this optimal cutoff of the utilization score, the NRBC and volume of cord blood collected was different between groups: the mean volume was 96.84 mL (±22.42) in group A and 123.09 mL (±27.69) in group B and there were 6.4 NRBCs/100 leukocytes (± 8.22) in group A and 10.96 NRBCs/100 leukocytes (±13.8) in the group B, as shown in Table 3.
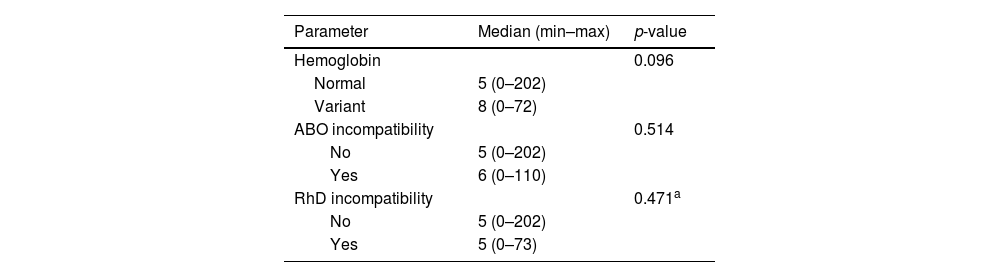
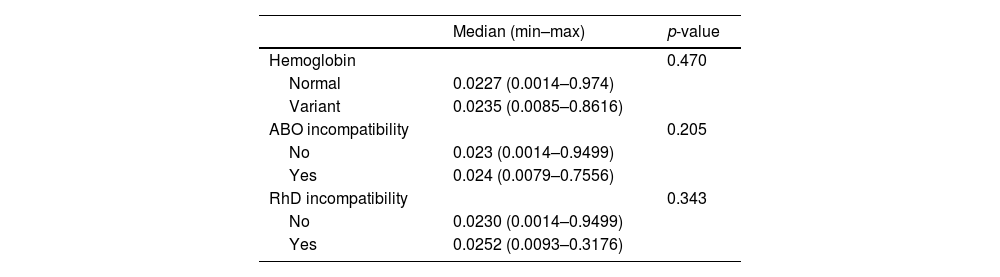
The CBUs were divided into two groups according to electrophoresis and presence of incompatibility. Group one showed normal hemoglobin and the absence of incompatibility, and group two, variant hemoglobin or the presence of any incompatibility. No difference was observed comparing theses parameters with the number of NRBCs or utilization score, as described in Tables 4 and 5.
Correlation of nucleated red blood cells and presence of variant hemoglobin, presence of ABO or RhD incompatibility.
| Parameter | Median (min–max) | p-value |
|---|---|---|
| Hemoglobin | 0.096 | |
| Normal | 5 (0–202) | |
| Variant | 8 (0–72) | |
| ABO incompatibility | 0.514 | |
| No | 5 (0–202) | |
| Yes | 6 (0–110) | |
| RhD incompatibility | 0.471a | |
| No | 5 (0–202) | |
| Yes | 5 (0–73) |
Mann–Whitney test.
Correlation of utilization score and presence of variant hemoglobin, presence of ABO or RhD incompatibility.
Mann–Whitney test.
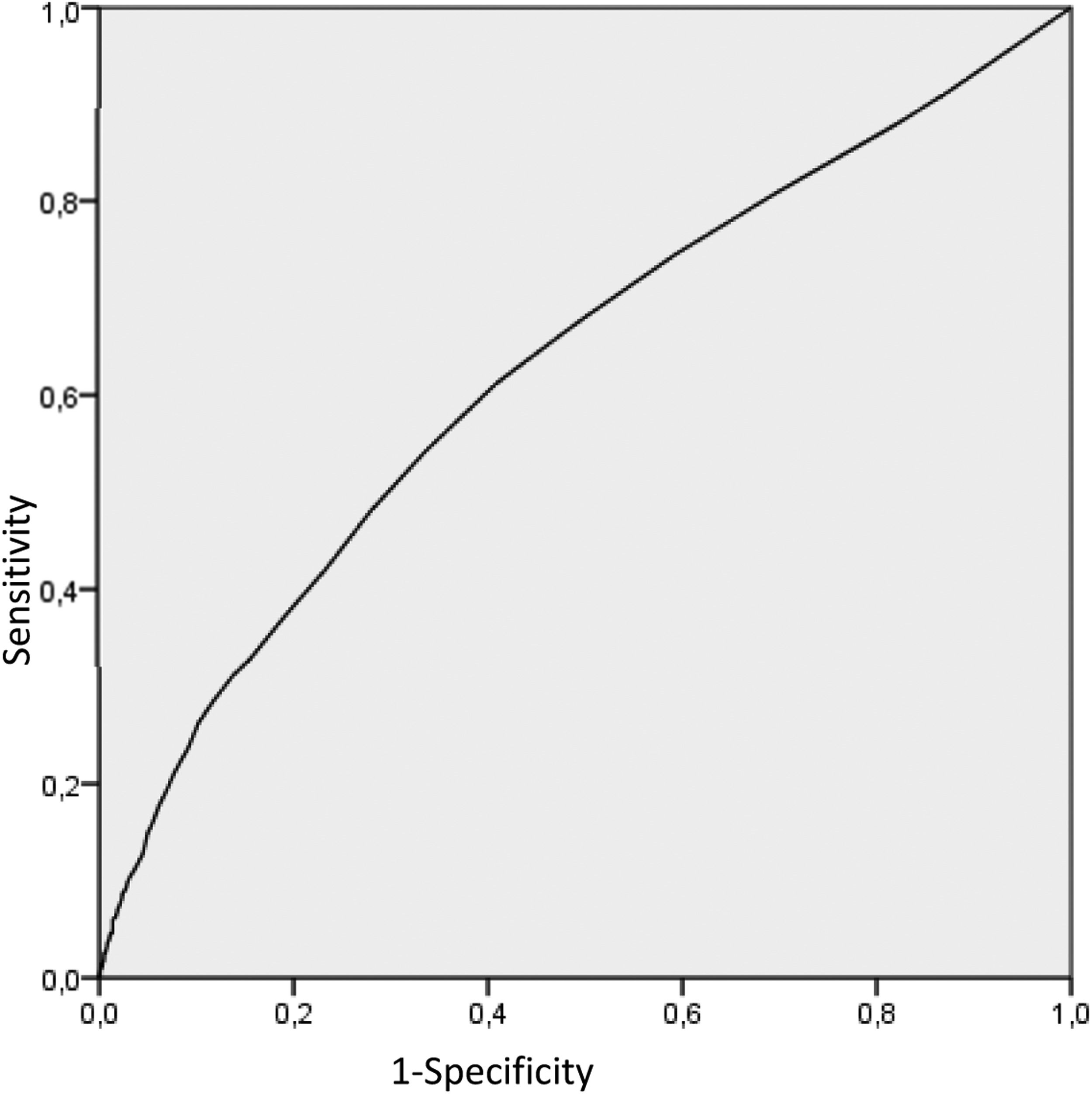
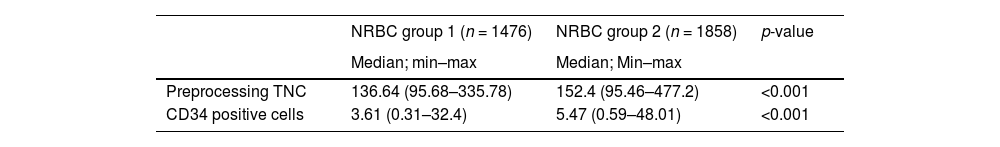
We performed an ROC analysis of the NRBCs based on the utilization score, trying to identify a cutoff number of NRBCs that correlated better with the probability of use. Even though the correlation of the NRBC and the score was weak, we found 5 NRBCs/100 leukocytes, with a 67.3% sensitivity and 51.2% specificity, that should be considered as a screening parameter, shown in Figure 1. Considering this cutoff, we selected 1858 units that are correlated with better quality parameters, as described in Table 6.
DiscussionCord blood is an important source of progenitor cells, particularly for transplantation in pediatric patients or in a poorly represented population in voluntary donor groups. Several public cord blood banks, distributed in several countries, were created with the aim to collect alternative HPCs for BMT.
Although the CBU seems to be good alternative source of HPC, a higher rate of graft failure can be observed, due to the lower dose of HPCs infused.22 Several studies have identified that total amount of nucleated cells, added to the total number of positive CD34 cells, post-thaw viability and in vitro expansion clonogenic assays have a better impact on unit grafting determination.3,5,23 Page et al. created and validated an Apgar Score for cord blood units based on total nucleated cells, CD34 positive cells, CFUs, mononuclear cell content and volume that correlates with neutrophil engraftment.9 Other studies evaluated some parameters that should be considered in the CBU graft and a guideline has been proposed for the CBU use for BMT based on the number of TNCs, HLA match, amount of CD34 cells and potency assays.24
In general, banks depend on the use of their units to be financially viable. Over the past few decades, the decreasing use of units has made the maintenance of cord banks a new challenge for their managers. Based on these quality parameters of the CBU, some studies have tried to determine which graft factors should be considered for the cryopreservation and storage of units, looking for a cost-effective strategy to maintain the cord blood bank sustainability. Considering the optimal cell dose suggestion of 2.5 × 107 TNC/kg for CBU transplantation, Querol et al. proposed a minimal pre-freezing level of 9 × 108 TNC for the United Kingdom public banks and the need to discard 45% of the units collected.25 Magalon et al. analyzed the CBUs registered in the Bone Marrow Donor Worldwide (BMDW) and evaluated their utilization score in a derived model based on multivariate analysis. They concluded that each additional unit of TNC (1 × 108) and CD34+ (1 × 106) was associated with an odds ratio of 1.212 (confidence interval 1.186–1.238) and 1.041 (confidence interval 1.021–1.061), respectively, for increased utilization and they found that by using a pre-freezing level of 18 × 108, the number of TNCs would be the best strategy with the lowest financial deficit for the bank.10
This study calculated the utilization score, based on the Magalon et al.’s study, and identified 19.4% of our cord blood bank inventory with a higher utilization score, however only four units in this inventory were transplanted.
We found a correlation of NRBCs with the engraftment capacity, which could be a previous screening before processing and cryopreservation. Stevens et al. evaluated patients who underwent CBU transplantation and observed that the units with highest amount of NRBCs had a correlation with a greater amount of TCNs, CD34 positive cells and a greater number of CFUs (R2 = 0.21; p < 0.001, R2 = 0.27; p < 0.001 and R2 = 0.22; p < 0.001, respectively) and it was a prognostic factor of neutrophil engraftment.27 Based on this study, cord blood banks have adopted the practice of adding NRBCS to the total number of nucleated cells. In our study, there is a weak correlation of NRBCs in the CBU with the utilization score (R2 = 0.245), probably reflecting the NRBC correlation with CD34 positive cells (R2 = 0.413).
There is still doubt about the mechanism by which the NRBC can influence the engraftment of the CBU. The literature evaluating the newborn with fetal distress demonstrates that a higher rate of NRBCs in the umbilical cord blood could be associated with situations, such as neonatal hypoxia, hemolytic diseases, pregnant women with preeclampsia, prolonged labor and gestational diabetes, reflecting a greater activity of erythropoietin.6,15,28,29 Lim et al. also identified the correlation between stress during delivery and higher numbers of nucleated cells, granulocytes, CD34 positive cells and hematopoietic progenitor cells in the umbilical cord blood, possibly by the mobilization by endogenous cytokines, improving the proliferative potential of the CBU.30
Although highly associated with pathological situations, cord blood has a normal NRBC number, probably reflecting greater hematopoietic activity in this period. Hanion–Lundberg et al. studied more than 1000 cord blood samples from single, uncomplicated pregnancies and showed a median NRBC of 8.55/100 leukocytes ± 10.27 (0–89), with no difference between samples from smoking mothers, anemia, use of illicit drugs or type of delivery.31 In our study, clinical screening was enough to select donors without hemolytic disease or the presence of variant hemoglobin.
In order to reduce CBU costs, public banks could use NRBC determination to screen better units that will be processed and cryopreserved. In our ROC analyses of the NRBC and utilization score, the correlation is weak; however, we identified a cutoff of 5 NRBCs/100 leukocytes with a 67.3% sensibility and 51.2% specificity that should be considered as a screening parameter. If this screening strategy were used at our cord blood bank, it would reduce the number of cryopreserved units to 1858 (55.5%), with 45% of our inventory with a higher utilization score and units with a greater number of TNCs and CD34 positive cells. Galel et al. also evaluated the NRBC as a screening parameter for the CBU and showed a correlation between the NRBCs and the amount of CD34 positive cells.26 In our inventory, using NRBC screening would increase the median of CD34 positive cells from 5.61 × 106 to 9.09 × 106 (p < 0.001) and TNCs from 156.12 to 205.9 × 107 (p < 0.001). The outcome with transplanted CBUs could not be evaluated in this study to correlate this screening parameter with the result in the clinical application of the inventory selected by this score.
ConclusionOur study shows that the number of NRBCs could be a feasible parameter for CBU screening, as it is associated with a greater number of TNCs and CD34 positive cells. Selection of the CBU with a high probability of use can contribute to the financial stability of the cord blood bank, reducing costs of processing, cryopreservation and maintenance of units. The quantification of NRBCs is already performed in all units and our minimum cutoff of 5 NRBCs/100 leukocytes can be a strategy, along with the TNCs, to better identify units for cord blood bank sustainable.
This study did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.