Sickle cell disease (SCD) is an inherited and multisystem blood disorder characterized by hemolytic anemia, vaso-occlusive crises (VOCs), progressive multiorgan damage and increased mortality. In Brazil, it is one of the most common monogenic diseases afflicting 60,000 to 100,000 individuals, however, there are sparse epidemiological data, as well as information on the utilization of public healthcare resources. Method: This was a 5-year (2016 - 2020) retrospective study conducted at one Brazilian reference center on SCD - Santa Casa de Sao Paulo, in Sao Paulo, Brazil.
ResultsAmong a total of 100 eligible adult patients, the median age was 31.0 years old, 84% of the patients were aged between 18 and 45 years old; 59% were women and 91% presented the genotype HbSS. The number of hematologist and non-hematologist visits at the outpatient unit were 2,198 and 1,436, respectively. The number of hospital ER visits was 758, of which 51% required 864 days of hospitalization. The main cause for seeking hospital medical care was the VOCs. The numbers and ratios of VOCs were: 1 to 10 VOCs, 64%; 11 to 20, 15%, and; 21 or more, 1%. There was a statistically significant difference between the number of VOCs and hospitalizations, as well as infection. Conclusion: Results indicate the burden of SCD on Brazilian patients’ daily lives, the impact of VOCs on public healthcare resources, the importance of having a national surveillance program to improve resource utilization and clinical outcomes of patients with SCD and the urgent need for the revitalizing of the current national comprehensive SCD care programs.
Sickle cell disease (SCD) is an inherited disorder of hemoglobin (Hb) characterized by chronic hemolysis and vaso-occlusive episodes, resulting in severe pain, progressive multiorgan damage and detrimental effects on the psychosocial well-being of those affected, their families and communities. Despite being the first monogenic disease described in the scientific literature over a century ago, SCD remains an illness with high morbidity and early mortality.1-2 The SCD is the most common inherited disorder worldwide, with well over 300,000 children being born with SCD yearly, 75% of whom are born in Africa.3 Based on the Brazilian neonatal screening program, between 2014 and 2020, the annual average of new cases of children diagnosed with SCD was 1087, with an incidence of 3.78 per 10,000 live births and an estimated number of 60,000 to 100,000 individuals living with SCD in Brazil.4,5
The pathophysiology of SCD is a result of HbS in low oxygen conditions, giving rise to rigid and fragile sickle-shaped red cells with impaired oxygen-carrying capacity.1,2 The Hb polymerization leads to hemolysis, anemia and vaso-oclusion. The sickling and hemolytic characteristics of sickle red blood cells (RBCs) incite an inflammatory cascade through interactions with the endothelium, white blood cells and platelets. Recurrent RBC sickling and hemolysis, combined with endovascular inflammation, result in acute and chronic organ damage at the cellular level, associated with acute, unpredictable and potentially life-threatening complications.6-8
The SCD is a group of inherited disorders, including sickle cell anemia (Hb SS), responsible for the most common genotype and most severe variant of SCD. Vaso-occlusive crises (VOCs) are one of the hallmarks of SCD, the most frequent complication among children and adults, and a substantial cause of morbidity, hospitalization and increased mortality.8,9
The management of SCD patients may include using hydroxyurea (HU), folic acid, blood transfusion, iron chelation, antibiotic therapies, vaccination, hematopoietic stem cell transplantation (HSCT) and gene therapy.2,8,9 The HU is an inhibitor of the ribonucleotide reductase drug with many beneficial effects for treating people with SCD, including: increasing fetal Hb concentration in RBCs, improving nitric oxide metabolism and reducing red cell-endothelial interaction and erythrocyte density.2,7,9,10 Such disease-modifying effects have been shown to decrease VOCs, acute chest syndrome (ACS), the number/length of hospitalizations and the need for transfusions, noticeably reducing the mortality rate and improving overall survival.9,10 Blood transfusion currently represents a supportive therapy to manage anemia, vasculopathy and VOCs and to prevent serious complications, particularly the risk of primary and secondary stroke.1,2,9 The HSCT is a potentially curative procedure, although its use is restricted to a few patients due to the high cost, toxicity and limited availability of suitable donors.1,7 In addition to HSCT, gene therapy is being explored as a curative option for SCD. The goal of gene therapy is to replace a patient's abnormal gene with new genetic material; it offers a potential cure for SCD without the need for a bone marrow donor or the toxicity associated with traditional conditioning regimens for HSCT.7
In the last two decades, the availability of mouse models of SCD has allowed both the characterization of the pathogenesis of sickle cell-related organ damage(s) and the identification of new pathophysiology-based therapeutic options, in addition to HU. These include agents, such as l-glutamine, crizanlizumab and voxelotor.7
Considering the high frequency of SCD in Brazil, the lack of a national SCD registry, the paucity of epidemiological data on the burden of disease on patients' daily lives and the impact of VOCs on public healthcare resources in our country, the objective of this study was to provide a real-life evidence data with a clearer picture of SCD in our country.
ObjectivesThe aim of this study was to describe the sociodemographic and genotype data and information on the use of the public healthcare resources (hematologist and non-hematologist visits, hospital emergency room (ER) visits and hospitalizations) of SCD patients during the five years of observation.
Material and methodsStudy typeThe current study was a retrospective cross-sectional descriptive study conducted at one Brazilian reference center on SCD at Santa Casa de Sao Paulo (SCSP) in Sao Paulo (southeastern Brazil) during a 5-year observation period (2016 - 2020).
Study participantsSCD patients had to meet all of the following criteria to be enrolled in this study: male or female patients 18 years old or over, diagnosis of SCD (genotype HbSS, HbSC and HbSβ0/+-thalassemia), with at least 3 outpatient visits during the first year of the observation period and written informed consent obtained before any study procedures. Patients with less than 3 outpatient visits in the first year of the follow-up were excluded from the study.
DesignThe SCD patient data was obtained from the patients’ electronic medical records at the hospital, preserving the confidentiality of information. A random sampling method was used to enroll the patient in the study and minimize the selection bias. The eligible patients were initially identified based on the medical records review in the site, with a unique code being attributed to each patient. Using the Excel randomization function (RAND) in all sheets, random samples were selected sequentially. The research was approved by the Research Ethics Committee of the Hospital (CAAE nº 51843721.6.0000.5479).
Definition of VOCsThe VOCs were defined as acute onsets of pain crises for which there is no medically determined explanation other than vaso-occlusion, which requires hospital medical care and therapy with parenteral opioids and/or nonsteroidal anti-inflammatory drugs. Different complicated crises, such as acute chest syndrome, priapism and hepatic or splenic sequestration, could also be classified as VOCs.
Data analysisThe descriptive analysis of the data was performed with the Statistical Package for Social Sciences software (SPSS, version 20.0, Chicago, IL, USA) and expressed as percentages, minimum and maximum values, median and mean. The Mann-Whitney test was used for continuous variables and the Chi-square or Fisher's exact test provided categorical variables. The significance level was 5% for the decision on differences between groups.
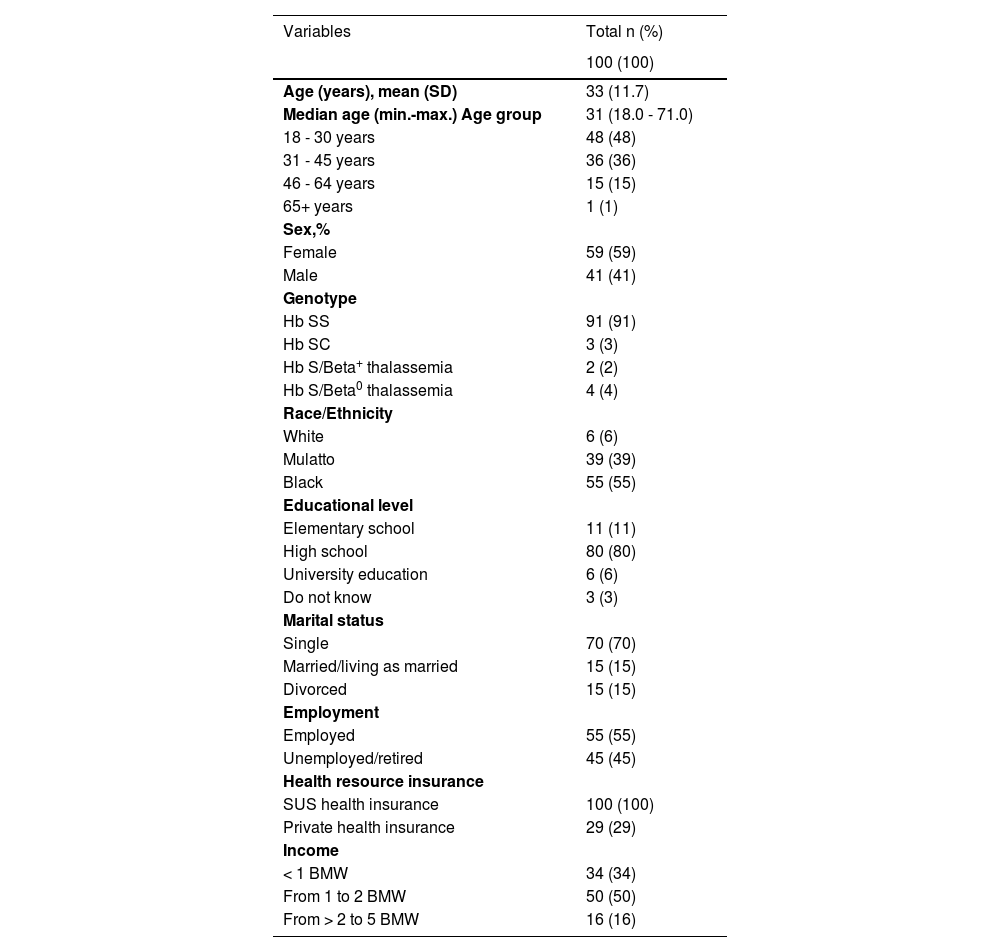
ResultsOne hundred and five patients were included in the study database. Five patients did not meet the eligibility criteria and were excluded. Thus, a total of 100 patients were enrolled and analyzed. The sociodemographic and genotype profiles are summarized in Table 1.
Patient sociodemographic characteristics and genotype.
SUS, public health system; BMW, Brazilian minimum wage.
The majority of patients (84%) were between 18 and 45 years old and only one patient was 60 or over; 59% were females and most patients declared themselves as black (55%) or mulatto (39%). The Hb SS genotype was the most common SCD genotype (91%) and 80% and 6% of the patients had high school and university education levels, respectively. Forty-five percent were unemployed/retired; 100% of the patients used public health insurance (SUS), although 29% also had private health insurance, and; 84% received 2 or less Brazilian minimum wages.
The drug HU was prescribed to 71 patients (71%), 75% received a single dose of 20 to 25 mg/kg/day and 35%, between 25 and 35 mg/kg/day. The indications for HU treatment included a history of VOCs (≥ 3 crises) that required medical support, recurrent ACS, stroke, recurrent priapism and severe persistent anemia (Hb < 7 g/dL) in the previous 12 months. Regarding blood transfusion, all patients had received at least one transfusion during their lives and approximately 27% were on a chronic packed RBC (pRBC) transfusion regimen, mainly due to secondary prevention of stroke. As for the iron chelation therapy, 17% were using deferasirox regularly.
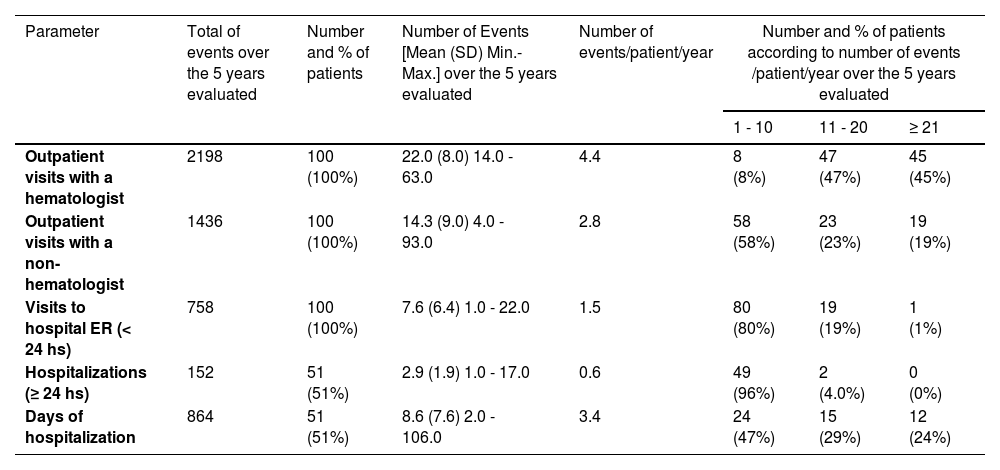
Over the five years evaluated, the number of hematologist and non-hematologist visits to the outpatient unit were 2198 [mean (SD) of 22.0 (8.0), approximately 4.4 visits/patient/year] and 1436 [mean (SD) of 14.3 (9.0), approximately 2.8 visits/patient/year], respectively. The most common non-hematologist visits included: ophthalmologist, gynecologist, urologist, cardiologist, surgeon/angiologist, endocrinologist, dentist and psychologist.
The number of hospital ER visits was 758 [mean (SD) of 7.6 (6.4), approximately 1.5 visits/patient/year], of whom 49% were discharged in less than 24 h with partial or complete resolution of the pain or with a prescription of analgesia and/or antibiotic therapy. Fifty-one percent of the patients required 864 days of hospitalization (mean (SD) of 8.6 (7.6), approximately 3.4 days/patient/year). (Table 2) In more than half of the hospitalizations, the patients received pRBC transfusions.
Distribution of healthcare resource utilization during the study period (2016–2020).
ER, emergency room; SD, standard deviation.
The leading causes for seeking hospital medical care were VOCs alone (46% being 70% due to painful crises, 22% ACS and 8% priapism), VOCs and infection (35%) followed by infection alone (19%). Most of the infections were related to the airways (sinusitis, tonsillitis and pneumonia) and urinary tract. The number of hospital ER visits due to VOCs (with or without infection) was 614 (81% of 758 events) and all 100 patients had at least one VOC during the study period. The numbers and percentage of VOCs/patient/year over the 5 years evaluated were: 1 to 10 VOCs, 65%; 11 to 20, 15%, and; 21 or more, 1%. Ten percent of the patients had 3 or more VOCs every year in the five years of the study.
There was a statistically significant difference between the number of VOCs and hospitalizations. Among patients with 1 to 3 VOCs and those with 4 or more VOCs, the average number of hospitalization days was 11.5 and 18.3, respectively (p = 0.003). A statistically significant difference was observed between VOCs and infection; patients with 1 to 3 VOCs had an infection rate of 19%, while those with 4 or more VOCs had 44% (p = 0.027).
DiscussionSociodemographic and genotype dataThe sociodemographic and genotype data observed in our study clearly highlight the severity of SCD, evidenced by the predominance of the Hb SS genotype, which is associated with the more severe clinical course, and the low life expectancy of patients since, in a cohort of 100 patients, only 1 was older than 60 years of age.
The HbSS genotype is the most frequent genotype in Brazil, although its prevalence may vary according to the Brazilian state. Results of a clinical and genetic ancestry profile of a large multi-center SCD cohort in Brazil analyzed a total of 2795 participants at six sites in 4 Brazilian states (Minas Gerais, Pernambuco, Rio de Janeiro and São Paulo) and observed that the Hb SS genotype was the most common SCD genotype (70.7%), followed by HbSC (23%), Sβ0thalassemia (3.0%) and Sβ+thalassemia (2.9%).11
In our current study, SCD mainly affected patients of Afro-descendant ethnicity, with low educational levels, as well as low per capita income, which is usually associated with greater socioeconomic vulnerability, worse health conditions and more difficult medical access. The vast majority were single, which undoubtedly reflects the disease's negative impact on the patient`s self-care and family dynamics and on the quality of life from the psychological perspective, which is worse in SCD patients who live alone. All these data were in accordance with other Brazilian studies.11-13
The current study observed that 45% were unemployed/retired, showing the great impact of the disease concerning this setting. In Brazil, there is limited information available specifically on the employment status of individuals with SCD. Two studies observed a rate of unemployed SCD patients ranging from 31% to 68.8%.11-13 The unpredictable nature of SCD symptoms, including fatigue and VOCs, and the need for frequent medical visits and hospitalizations can make it challenging for patients to maintain regular employment. It is important for employers and policymakers to recognize the unique challenges faced by SCD individuals and provide supportive measures, such as flexible work arrangements, to ensure their inclusion in the workforce.14
Regarding health resources, 100% of the patients used public health insurance (SUS), although 29% also had private health insurance. These data highlight the importance of the public health service in caring for SCD patients. Patients with private insurance coverage usually have easy access to healthcare utilization (laboratory and imaging tests, consultations with doctors of different specialties, emergency rooms, and hospitalization) which can positively influence the control of the underlying disease.14 Interestingly, even those with private health insurance continue to be followed at our service. One of the explanations for this is likely due to the security and better healthcare that patients may perceive by being followed up at referral services for SCD patients, remembering that most of these centers are located in the public health sector.
SCD treatment experiences: hydroxyurea, deferasirox and blood transfusionThe HU improves the clinical severity and the hematological parameters of SCD patients and reduces the morbidity and mortality rates of the disease, with an increase in survival. The two main aspects for achieving therapeutic success with HU are the appropriate dose and treatment adherence. Poor adherence is the primary reason HU therapy is ineffective in child and adult SCD patients.2,10 In our study, HU was prescribed to 71% of the patients. Despite the evidence of safety, efficacy and tolerability of HU in both pediatric and adult age groups, this significant therapeutic advance has been underutilized in Brazil and in many countries around the world. Lobo et al.15 analyzing 1144 patients with SCD at HEMORIO (Rio de Janeiro) observed that HU was prescribed to 40.5% of the children and 36.4% of the adults. Carneiro-Proietti et al.11 reported 458 children (29.3% of 1104) and 447 adults (36.3% of 1044) on HU at the time of enrolment. Barriers to using HU are related to the difficulty of accessing the drug, in addition to the patients, parents and healthcare providers’ fear of possible adverse effects on reproduction and fertility.1,2,10
Blood transfusion remains a supportive therapy for many complications associated with SCD, including abrupt worsening of anemia, vasculopathy, VOCs and organ dysfunction.2,7,8 The chronic pRBC transfusion regimen significantly reduces the risk of primary and secondary stroke in pediatric patients with SCD.2,8 Nevertheless, chronic pRBC transfusion inevitably leads to secondary iron overload that can cause significant damage to many organs, such as the liver, endocrine system and heart. It is associated with morbidity and mortality in SCD.2 As a result, guidelines currently recommend initiating iron chelation therapy once liver iron content increases to over 7 mg Fe/g dry weight, if steady state serum ferritin levels are over 1000 ng/l, or if patients have received cumulative transfusions of at least 20 pRBC units.1,2 The most used iron chelator in Brazil is deferasirox because it is effective, safe and can be administered orally once a day.16
In the current study, all patients had received at least one transfusion during their lives and approximately 27% were on chronic pRBC transfusions, mainly due to secondary prevention of stroke. Regarding iron chelation therapy, 17% were regularly using deferasirox. These data are in agreement with those observed in the literature.1,2,5,6
Public healthcare resource utilizationThe regular follow-up of patients at the hematology outpatient unit guarantees clinical and laboratory monitoring and the renewal of forms for accessing medicines dispensed by the SUS. The visits to non-hematologists for prevention and/or follow-up/treatment depend on the patient complications. In our study, we observed approximately 7.2 visits to the outpatient unit and 1.5 visits to the ER hospital unit per patient/year, respectively, and 3.4 days of hospitalization/patient/year in the 5-year follow-up. As with any chronic disease, it is expected that individuals living with SCD have a frequent need for healthcare assistance. There is no available specific information on the comparison of the healthcare resource utilization between SCD and other chronic diseases. We do know that patients with SCD have higher rates of acute care utilization, hospitalization and readmission, compared to the general population.16
The VOCs are the leading cause of morbidity, hospitalization and increased mortality among SCD patients1,2 and it is estimated that approximately 30% of SCD patients have at least three VOCs per year15-17 In the current study, 100% of the patients had at least one VOC, a number of VOCs/patient/year of 1.5, 20% with 11 or more VOCs/patient/year and 10% had 3 or more VOCs every year in the five consecutive years of the study. These data strongly suggest the negative impact that the disease has on the SCD patient, as well as the high utilization rate of healthcare resources, and are in accordance with the data published in the literature.15-17 It is important to emphasize that Brazil is a continental country with significant regional differences in the distribution of SCD patients, social inequalities and access to specialized healthcare, facts that may explain or contribute to the high morbidity and mortality rates in our country.
A statistically significant difference was observed between the numbers of VOCs, as well as infections, and hospitalizations. Infection is a major determinant of the outcome in patients with SCD and is the most important cause of premature deaths among children.1,2 Splenic dysfunction has a crucial role in the increased susceptibility to bacterial infections (pneumococcal and Haemophilus influenza infections), particularly in children with SCD, suggesting that basic interventions, such as neonatal screening, immunization programs, bacterial prevention and comprehensive healthcare management could lead to substantial improvement in survival among child and adult patients with SCD.1,2,5,18
Although we had no deaths in our studied population between 2016 and 2020, the life expectancy of patients with SCD is reduced by approximately 20 to 30 years, compared to the general population.2,5,6 Cancado et al.19 analyzed 6,553,132 deaths registered in Brazil from 2015 to 2019, of whom 3320 were individuals with SCD. Among them, the median age at death was 32.0 years old, 37 years younger than the general population (median age at death of 69 years old).
In addition to the high utilization of healthcare resources, it is important to consider the economic burden of the disease worldwide.1,2,6 We did not analyze this aspect in our study, however, Lobo et al. showed the high costs associated with acute care resource utilization among individuals with SCD at a single tertiary center in Rio de Janeiro (Brazil)15 and Silva-Pinto et al.16 showed that SCD patients might generate an annual economic burden for Brazilian society of approximately 400 million USD. These data highlight the importance of strategies to better control the disease to reduce its impact on patients and society.
Although it does present important information on Brazilian SCD patients, our study had limitations. First, we only included data from one center in Brazil, limiting the generalizability. Second, we only analyzed admissions to our hospital (SCSP). Due to the distance between the patient's home and a reference hospital, or because he or she has private health insurance, the patient may seek medical care closer to home. Therefore, the hospital ER visits and hospitalizations rates may be even greater than those which were reported in the current study. The strength of our study lies in its scope, in that it covers the burden of SCD on Brazilian patients’ daily lives, providing real-life evidence data with a clearer picture of SCD in our country.
Despite multiple statements and calls to action from the World Health Organization (WHO) to recognize and address the global burden of SCD, there remains a crucial paucity of data in individual countries or across the highest-burdened regions of the world to accurately quantify this problem and drive resource allocation to improve the diagnosis, management and awareness of SCD. Systematic data collection for SCD through population-wide surveillance, an initiative already taken up in several countries, and of which Brazil must be part, can help to facilitate progress in the treatment and different types of registries and databases can be complementary to the surveillance data, promoting an economy of scale in resource allocation.20,21
ConclusionThese results emphasize the burden of SCD on Brazilian patients’ daily lives, the impact of VOCs on public healthcare resources, the need for improved therapeutic options to limit or prevent disease progression, the importance of having both a longitudinal clinical registry and a national surveillance program to improve resource utilization and clinical outcomes of patients with SCD and the urgent need to revitalize the current national comprehensive SCD care programs.
ContributorsAll authors contributed equally to the writing of the manuscript and approved the final version.