The Hb Deer Lodge (β2 His>Arg; HBB:c.8A>G) is a structural hemoglobin variant described in some populations around the world, characterized by increased oxygen affinity, but does not confer clinical symptoms to its carriers. The coinheritance of the Hb Deer Lodge with the most common hemoglobin variant, Hb S, has been reported only once; however, functional data were not described. Here we show a case of the Hb S and Hb Deer Lodge carrier in heterozygosity.
MethodsThe Hb S and Hb Deer Lodge association was identified by High-Performance Liquid Chromatography (HPLC), reverse phase HPLC and the β globin gene sequencing. The functional characterization of this interaction was obtained using the O2 dissociation curve, determination of the cooperativity between the globin chains and the Bohr effect in the presence and absence of organic phosphates.
ResultsWhen the Hb S and Hb Deer Lodge were associated, there was a decrease in cooperativity, no significant changes in oxygen affinity and no significant Bohr effect changes.
ConclusionDespite these genetic variations, the carrier showed no hematological alterations and no clinical symptoms, possibly due to the high oxygen affinity of the Hb Deer Lodge, which interferes with the Hb S polymerization.
Hemoglobinopathies are characterized by genetic alterations in the globin chain genes that lead to structural modifications in the hemoglobin molecule. Currently, more than 900 mutations in the beta-globin chain gene have been described.1 Among them, the mutation leading to the formation of hemoglobin (Hb) S is the most frequent in the general population. The amino acid substitution of glutamic acid for valine (β6 Glu>Val; HBB: c.20A>T) allows the hemoglobin polymerization when deoxygenated.2 This modification in compound heterozygosity leads to a clinical condition known as sickle cell disease (SCD), which presents heterogeneous symptomatology, depending on the associated hemoglobin variant.3
The Hb Deer Lodge (DL) is a structural variant of hemoglobin resulting from a point mutation in codon 2 of the beta-globin chain (β2 His>Arg; HBB:c.8A>G), which is characterized by increased oxygen affinity, but does not confer any symptomatology to its carries.4 The Hb DL was first described in a man with Welsh-Dutch-English ancestry5 and was later found in a Caucasian American woman6 and Black4 and Venezuelan7 families. However, this hemoglobin association with the Hb S was only reported once, in 1976, in the USA,4 which makes our study the first to report, describe and analyze this interaction through functional properties.
MethodsThe carrier of these variants is a female child treated at the local hematology reference center (the Hospital of Hematology and Hemotherapy Foundation of Pernambuco - HEMOPE). After obtaining informed consent, blood samples were collected from the child and family members in tubes with EDTA as an anticoagulant and no anticoagulant for hematological and biochemical parameters, respectively. Hematological parameters were measured using an automated cell counter, Coulter STKS (Coulter Electronics, Hialeah, FL, USA), and the erythrocyte morphology was analyzed. Biochemical parameters were estimated using the Cobas C501 analyzer (Roche Diagnostics (®), Meylan, France). The electrophoresis of hemoglobin at an alkaline pH in cellulose acetate was executed and was followed by the solubility test to confirm the presence of Hb S. The hemoglobin profile was investigated by cation-exchange high-performance liquid chromatography (HPLC, Variant II™, Bio-Rad Laboratories, Hercules, CA, USA) and reverse phase HPLC using the System Alliance 2695 (Waters, Milford, MA, USA) according to the manufacturer's instructions.
Following the DNA extraction by a standard phenol-chloroform method, the beta-globin gene was amplified and sequenced in the ABI PRISM® 3500 Genetic Analyzer to identify the unknown hemoglobin.
The functional characterization was performed by the Rossi-Fanelli and Antonini8 chemical kinetics method, using the O2 dissociation curve, determination of the cooperativity between the globin chains and the Bohr effect, in the presence and absence of organic phosphates.9 The Bohr effect values of the Hb DL and Hb S – DL interaction were compared to normal Hb A. The O2 affinity was evaluated by determining the p50 value, which estimates the partial pressure of O2 that results in the 50% saturation of hemoglobin; therefore, the higher the affinity for O2, the lower the p50 value. The heme-heme cooperativity was calculated using the Hill coefficient (n), for which values less than or greater than 1 represent, respectively, negative or positive cooperativity. The protein was purified by the Sephadex G25 to obtain its most purified (stripped) state. Such kinetic studies were performed at 25°C, at a pH between 6.5 and 8.5, using a 50 mM HEPES buffer. Tests to study the hemoglobin interaction with oxygen in the presence of an allosteric effector with a function similar to 2,3 – diphosphoglycerate (2,3 – DPG) were performed with inositol hexaphosphate (IHP) at a final concentration of 1 mM. The IHP has physical-chemical characteristics similar to those of 2,3 – DPG and binds to the same active site with a greater affinity for hemoglobin, providing higher structural stability to the molecule. The previously described methods were performed for the patient and her father to compare the Hb DL in heterozygosity with the Hb S and its interaction with the Hb A, respectively.
ResultsThe female patient, born in 2007 in Pernambuco, a state in northeastern Brazil, presented cyanosis at birth, requiring artificial oxygenation for 2 hours. She had compensated microcytosis and hypochromia. After performing the newborn screening test (Hb F = 78.8%; Hb A2 = 11.2%; Hb S = 10.0%), the patient was diagnosed with a hemoglobin variant and was referred to the local specialized hematology center. The alkaline electrophoresis subsequently revealed migration of two bands slower than Hb A (one of them an S-like, confirmed by the solubility test). The hemoglobin quantification by cationic exchange HPLC showed 7.9 % of fetal hemoglobin (Hb F), 38.1% of Hb S and 54.0% of an unknown hemoglobin co-eluent to Hb A2. The chains were isolated by reverse-phase HPLC. The area of absorption peaks showed 10.73% of Heme, 24.05% of globin βS, 18.19% of globin β Deer Lodge and 47.04% of α globin.
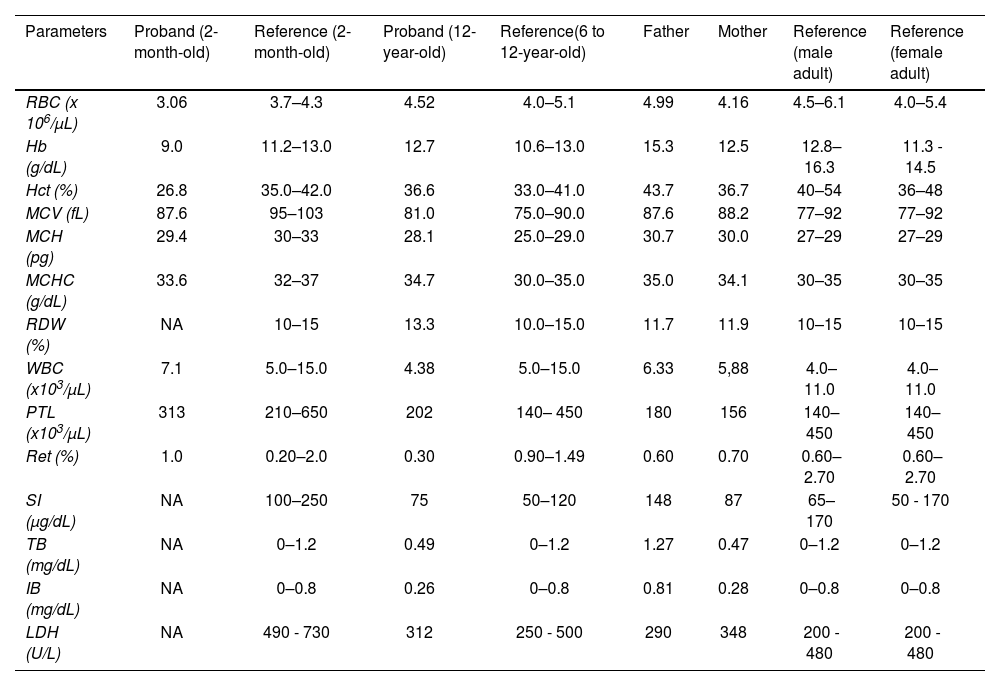
The sequencing of the β globin gene revealed a mutation at codon 2 (β2 His>Arg; HBB: c.8A>G) in heterozygosity, characterizing the Hb Deer Lodge, as well as a mutation at codon 6 (β6 Glu>Val; HBB: c.20A>T), relative to the Hb S. The familial molecular study revealed, both in heterozygosity, the Hb S mutation in the mother and the Hb Deer Lodge in the father. Table 1 describes the hematologic profiles of the patient, obtained at two months old and 12 years old, as well as those of her parents. From the last clinical examinations at 12 years old, the child presented as asymptomatic, weighing 45.5 kg and measuring 161 cm, with the blood pressure at 120/60 and a eutrophic nutritional status (BMI percentile = 25).
Summary of hematologic and biochemical data of the patient and her family members.
RBC: red blood cells; Hb: hemoglobin; Hct: hematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; CHCM: mean corpuscular hemoglobin concentration; RDW: Red cell distribution width; WBC: white blood cells; PTL: Platelets; Ret: reticulocyte count; SI: serum iron; TB: total bilirubin; IB: indirect bilirubin; LDH: lactate dehydrogenase; NA: not available.
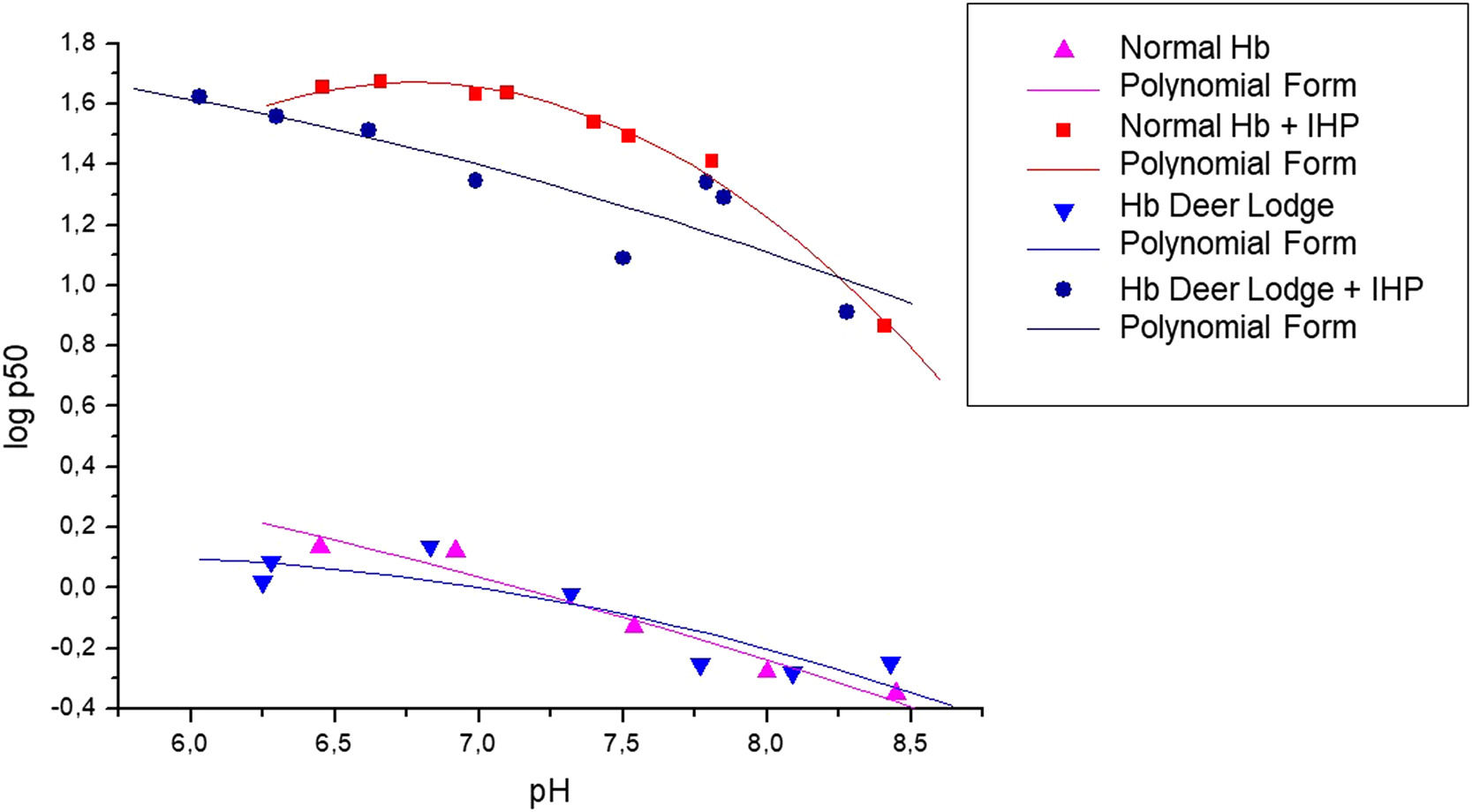
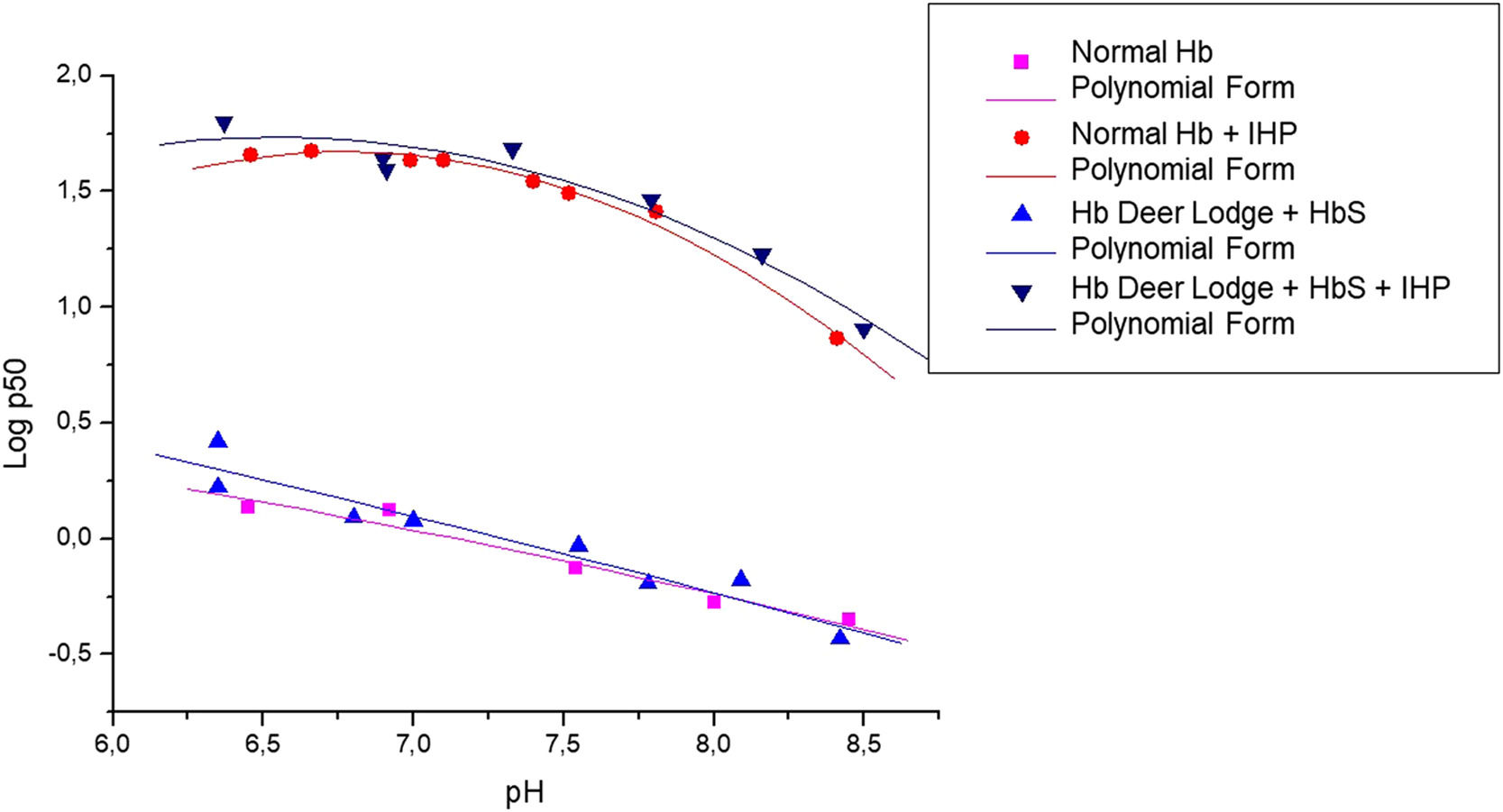
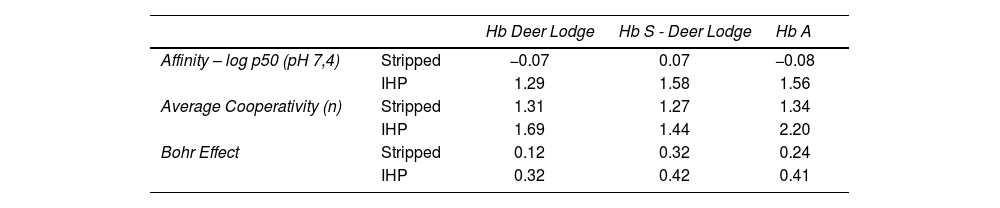
As can be observed in the graph obtained experimentally (Figure 1), the variant shows a significant decrease in the O2 dissociation curve, especially when IHP is added. Table 2 also shows that the addition of IHP promotes a greater reduction in the Hb DL cooperativity than Hb A, while the stripped state of Hb DL showed a significant reduction in the Bohr effect, compared to Hb A. When comparing the conjugation of the two variants (Hb S and Hb DL) with the Hb A curve, the dissociation curve of the Hb S-DL did not show a considerable change in oxygen affinity (Figure 2). However, the calculation of the heme interaction resulted in reduced cooperativity between globin chains when in the presence of IHP, which denotes a greater affinity of the Hb DL for O2. In the absence of IHP, this behavior was briefly reduced. The Bohr effect event did not show a significant variation with the addition of IHP, showing a greater variation when this molecule was absent. The values obtained in each analysis are described in Table 2.
Oxygen dissociation curves for hemoglobin Deer Lodge. Oxygen dissociation curve of hemoglobin Deer Lodge (obtained from the patient's father), compared to hemoglobin A (obtained from healthy blood donors with normal hemoglobin profile as control), in the presence and absence of inositol-hexaphosphate (IHP).
Quantitative results of affinity, cooperativity and Bohr effect for Hb Deer Lodge and Hb S – Deer Lodge association.
IHP: Inositol – hexaphosphate.
Oxygen dissociation curves for hemoglobin S – Deer Lodge association. Oxygen dissociation curve of hemoglobin S and Deer Lodge (Hb S + Hb Deer Lodge, obtained from the case patient), in the presence and absence of inositol-hexaphosphate (IHP), compared to the normal Hb A profile (obtained from healthy blood donors with normal hemoglobin profile as control).
The Hb Deer Lodge results from the replacement of histidine by arginine in the β2 position at the 2,3 – DPG binding site, causing conformational modifications in this region. The 2,3 – DPG acts as a hemoglobin allosteric effector, so the increase in its concentration decreases the hemoglobin affinity for oxygen and vice versa. The His → Arg substitution changes the density of positive charges at the 2,3 – DPG binding site, increasing the frequency and extent of conformational fluctuations in the deoxy structure, promoting increased O2 affinity10 and less affinity for 2,3 – DPG or IHP in this heterotrophic site.
In our experiments with Hb DL in heterozygosis, the oxygen affinity changes were better noticed when organic phosphate was added. The Hb DL mutation occurs at the 2,3 – DPG or IHP binding site; therefore, adding IHP in vitro promotes greater affinity for O2.11 Furthermore, since its binding site is altered in Hb DL, the IHP binds to Hb A, allowing it to release oxygen. Thus, the higher oxygen concentration leads to an increased affinity for the Hb DL variant. The Bohr Effect describes the oxygen affinity fluctuation according to the pH variance. Due to this property, the O2 binding to each heme group is cooperative, which means that the first oxygen binding enhances (positive cooperation) or reduces (negative cooperation) the affinity for the following O2 molecule until reaching the complete hemoglobin saturation.12 Here, we observed that the Hb DL, compared to the Hb A, has lower heme-heme cooperativity when IHP is added. In contrast, the Bohr effect of Hb DL in stripped state and with IHP addition was reduced, compared to Hb A, due to the hemoglobin affinity alteration according to the pH.
It was also observed that the Hb S does not interfere considerably with the Hb Deer Lodge affinity for O2 when in its purified state. However, with the addition of IHP, a reasonable decline in the hemoglobin DL affinity for O2 was noticed in concomitance with the presence of Hb S. Despite this reduction, the affinity was close to that presented by Hb A. Furthermore, the association of these mutations seems to normalize the Bohr effect when organic phosphate IHP is added – which tends to normality. In addition, although the cooperation between globins was positively influenced in both states, the Hb S – DL interaction revealed less positive cooperation, compared to the Hb DL in heterozygosis. Since the Hb S mutation does not affect the binding site of 2,3 – DPG, the increase of this molecule would lead to a higher O2 release by this variant.13 However, the Hb DL and its greater O2 affinity seemed to compensate this effect. Therefore, these two simultaneous variants demonstrated less structural mobility and interaction between hemes without negatively influencing affinity.
It is also interesting to note that the carrier of the Hb S - DL interaction clinically does not present sickle cell disease.14 This may be explained by the high affinity of Hb DL for oxygen, which interferes with the Hb S polymerization in its T-state (low O2 affinity) since the polymerization requires an environment with low O2 availability.15 As previously highlighted in a report on the association of Hb S with another variant with alteration in the 2,3 – DPG binding site, the Hb Abruzzo, hemoglobin with high oxygen affinity, may stabilize the hemoglobin S in the R-state (high O2 affinity), thereby inhibiting the molecule sickling.16
ConclusionIn summary, the S – DL combination cannot be distinguished from the sickle cell trait only by clinical and hematological analysis, as the latter has an abundance of Hb A in the erythrocytes, preventing the polymerization of Hb S, presenting, therefore, characteristics similar to those of the S – DL patient. In the same way that the association does not present functional and clinical changes, the hemogram of the patient also does not present any hematological alterations. Although these findings describe the functional profile of the S-DL interaction, the study is limited to the mutation rarity for further comparisons to different hemoglobin profiles and more complex analyses.