One of the most critical complications in myelodysplastic syndromes (MDS) is the progression to acute myeloid leukemia (AML). The dynamics of clonal evolution in MDS and how acquired mutations can be used as biomarkers to track disease progression remains under investigation.
Objective and methodHerein, we investigated the frequency of common myeloid clonal mutations (FLT3, NPM1, JAK2, IDH1 and IDH2) in 88 patients with MDS and 35 AML patients with myelodysplasia-related changes, followed at a single reference center in northeastern Brazil.
ResultsOverall, 9/88 (10%) of the MDS patients and 9/35 (26%) of the secondary AML patients had at least one mutation. While the JAK2 V617F mutation was the most frequent in the MDS patients, the FLT3, NPM1, IDH1 and IDH2 mutations were more frequently found in the secondary AML group. Furthermore, there was a higher frequency of FLT3, NPM1, IDH1 and IDH2 mutations in MDS patients classified as high-risk subtypes than in those of lower risk.
ConclusionDespite the limited sample size, our data suggest that mutations in FLT3, NPM1, IDH1 and IDH2 genes could be potential biomarkers to detect early disease progression in MDS.
One of the most critical complications of myelodysplastic syndromes (MDS) is the progression to acute myeloid leukemia (AML).1,2 Although many molecular abnormalities were linked to the development of MDS, the key events involved in its progression to AML remain poorly understood.2,3 In current clinical practice, both diagnoses of disease-progression and therapy decision are mostly based on manual count of bone marrow (BM) blasts. However, this approach is not only subject to interobserver bias, but also to variance in results due to the low number of analyzed cells.3–5
Recent reports evaluating the clonal dynamics in MDS identified newly acquired mutations with the potential to unleash clonal expansion and facilitate transition to AML,6–8 while others identified mutations associated with a lower risk of AML development. 8–10 Translating this knowledge into benefit for patients is challenging, but close monitoring of these molecular markers could allow for early diagnosis of disease progression. Herein we investigated the frequency of mutations previously reported to promote clonal evolution in myeloid malignancies, using a cohort of 88 patients with newly diagnosed MDS and 35 AML patients with myelodysplasia-related changes, followed at a single reference center in northeastern Brazil.
MethodsThe study adhered to the tenets of the declaration of Helsinki. All patients or their relatives gave their written informed consent for scientific evaluations and the study was approved by the Hospital Internal Review Board (#037/2010). Diagnostic bone marrow samples from adult MDS and AML patients with myelodysplasia-related changes prior to treatment were collected upon written informed consent, between the years of 2010 and 2016. Inclusion criteria for these two groups followed the WHO guidelines.11
For molecular analyses, genomic DNA from bone marrow samples was extracted using the Puregene kit (Gentra System), according to the manufacturer’s protocol. Detection of NPM1, and FLT3-ITD mutations were performed by conventional polymerase chain reaction, as described previously.12,13 Screening for IDH1 and IDH2 mutations were performed by Sanger sequencing with primers flanking the mutational hotspots of those genes (IDH1: codons R132 and IDH2: R140/R172). The product cleanup reagent (PCR) products were purified with ExoSAP-IT™ PCR Product Cleanup Reagent (Applied Biosystems). Sequencing reactions were performed with the BigDye Terminator v3.1 (Applied Biosystems) and run-in capillary electrophoresis (ABI 3500, Applied Biosystems). The JAK2 V617F mutations were evaluated by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP), as previously described.14
ResultsPatient’s baseline featuresFollowing the FAB classification system, 46 (52%) patients displayed refractory anemia (RA), 25 (28%), refractory anemia with excess blasts (RAEB), nine (10%), refractory anemia with ring sideroblasts (RARS), four (4.5%), chronic myelomonocytic leukemia (CMML), three (3%), hyperfibrotic MDS, and one patient (1%) could not be classified. Using the 2016 revision of the WHO classification,11 26 (30%) MDS patients presented multilineage dysplasia (MDS-MLD), 15 (17%), single lineage dysplasia (MDS-SLD), three (3%), MDS-ring sideroblasts with mutilineage dysplasia, five (6%), MDS-ring sideroblasts with single lineage dysplasia, 13 (15%), MDS with excess blasts 1 and 12 (13.5%), MDS with excess blasts 2. Of the remaining patients, six (7%) were MDS with isolated deletion in chromosome 5q (del(5q)), four (4.5%), CMML, three (3%), hyperfibrotic MDS, and one (1%) was unclassifiable. The mean age at diagnosis was 64 years (ranging from 21 to 88 years) and the male/female ratio was 0.95. The cytogenetic analysis was successfully performed for 35 (40%) patients. Overall, 25 patients (71%) were cytogenetically normal, seven had deletion of 5q (20%) and three patients had other alterations. Due to the high number of patients without cytogenetic data, the Revised International Score System (IPSS-R) could not be applied. With respect to the morphological findings in the bone marrow, 39 patients (44%) exhibited a hypercellular bone marrow, while 31 (35%) exhibited hypocellularity and 18 (21%), normal cellularity. Bone biopsy revealed the presence of reticulin fibrosis in eight patients (9%) and abnormal localization of immature precursors (ALIP) in seven patients (8%).
Additionally, 35 AML patients were included, 17 of whom had both a clinical history of MDS preceding the AML and overt multilineage dysplasia in ≥50% of BM cells at diagnosis. The other 18 had no previous history of MDS, but carried cytogenetic abnormalities that are recognized by the WHO classification system as sufficient to diagnose AML with myelodysplasia-related changes.11 The AML cases carrying one of these features, but with prior cytotoxic therapy for other diseases were not included. The mean age at diagnosis of the AML patients with myelodysplasia-related changes was 54 years, ranging from 22 to 87 years and the male/female ratio was 1.5. Cytogenetic analysis was successfully performed for 19 (54%) patients, twelve (63%) of whom had a complex karyotype, five, deletion of chromosome 7 (26%), one, del(11)(q23) and one del(8)(q22q24).
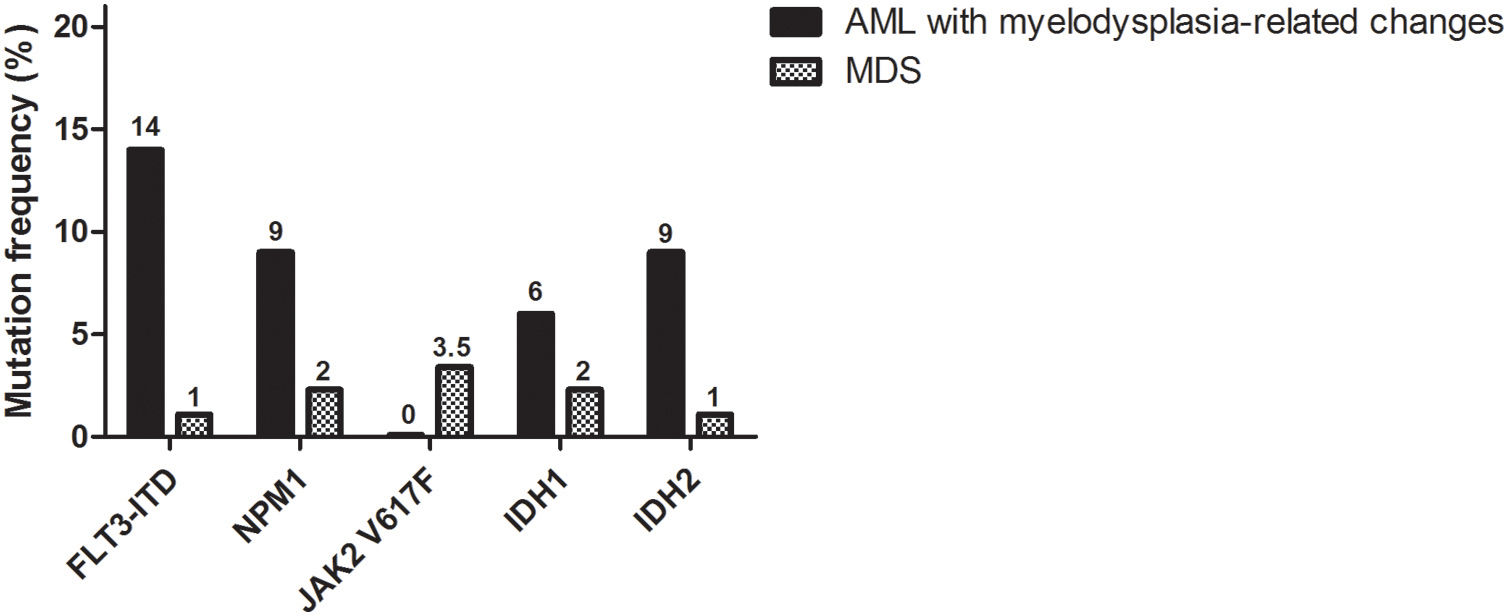
Screening mutationsMolecular analysis revealed that 9/88 (10%) MDS patients had one of the investigated mutations, the JAK2 V617F being the most frequently found mutation (three cases, 3.5%), followed by NPM1 and IDH1, detected in two patients (2%) and FLT3-ITD and IDH2, identified in one patient (1%) (Fig. 1). On the other hand, 9/35 (26%) of the AML cases with myelodysplasia-related changes had at least one of the investigated mutations, with a higher frequency of FLT3, NPM1, IDH1 and IDH2, whereas the JAK2 V617F was completely absent in this group (Fig. 1). In two AML patients, FLT3-ITD co-occurred with IDH2 R172K or IDH1 R132S. Baseline characteristics of patients harboring mutations are summarized in Table 1.
Characteristics of patients with gene mutations.
| Patient ID | Sex/Age | Classification | BM blasts (%) | Karyotype | Molecular abnormality |
|---|---|---|---|---|---|
| FAB/WHO | |||||
| MDS Patients | |||||
| P15 | F/78 | RAEB/MDS-EB-2 | 13 | N/A | IDH1 R132S |
| P30 | M/55 | RA/MDS-SLD | 1 | 46, XY | JAK2 V617F |
| P46 | M/57 | CMML/CMML-0 | 2 | N/A | JAK2 V617F |
| P50 | F/65 | AR/MDS-SLD | 2 | 46, XX | NPM1 |
| P68 | M/65 | RAEB/MDS-EB-1 | 9 | N/A | IDH2 R172K |
| P70 | F/63 | RAEB/MDS-EB-2 | 7 | N/A | JAK2 V617F |
| P77 | F/62 | RAEB/MDS-EB-1 | 6 | N/A | FLT3-ITD |
| P78 | F/75 | RA/MDS-MLD | 2 | N/A | IDH1 R132C |
| P88 | M/56 | CMML/ CMML-1 | 3 | N/A | NPM1 |
| AML with myelodysplasia-related changes | |||||
| P93 | F/64 | AML-MO | 85 | Complex karyotype | FLT3-ITD, IDH2 R172K |
| P94 | M/49 | AML-M2 | 66 | Complex karyotype | IDH2 R172K |
| P95 | F/71 | AML-M2 | 37 | Complex karyotype | IDH2 R140 |
| P105 | M/59 | AML-M1 | 25 | 46, XY, del(7)(q22q32) | IDH1 R132H |
| P106 | M/23 | AML-M7 | 42 | 45, XY, -7 | FLT3-ITD, IDH1 R132S |
| P111 | M/58 | N/A | N/A | NPM1 | |
| P112 | F/27 | AML-M4 | 43 | N/A | FLT3-ITD |
| P113 | F/43 | N/A | N/A | N/A | NPM1 |
| P114 | F/87 | AML-M1 | 83 | N/A | FLT3-ITD |
| P122 | F/63 | AML-M2 | 34 | N/A | NPM1 |
| P123 | F/50 | AML-M2 | 44 | N/A | FLT3-ITD |
Of interest, from the six MDS patients with mutated FLT3, NPM1, IDH1 or IDH2, three (50%) were MDS-EB1/2, while the frequency of these subtypes in non-mutated patients was 26% (21/82). However, the limited number of mutated MDS patients did not allow for further validation of this finding by statistical analysis.
DiscussionMutational profiling of MDS and AML subjects with myelodysplasia-related changes revealed a higher frequency of the mutations FLT3, NPM1, IDH1 and IDH2, but not JAK2, in the AML patients. Of note, among the MDS group, these mutations occurred more commonly in higher risk subtypes. Although preliminary, these findings point towards the role of these mutations in disease progression to AML and consequently, their potential use as biomarkers for this condition. Supporting current findings, another study using a similar approach found higher rates of IDH1/2 mutations in AML-progressed than in MDS cases, but did not investigate FLT3 and NPM1.15 Moreover, Makashima et al., and Meggerndorfer et al., showed that AML progression is mediated by molecular changes in a particular set of genes that includes FLT3, NPM1, IDH1 and IDH2.6,16
Interestingly, evidence from studies with conditional animal models suggests that some of these mutations, such as FLT3-ITD and NPM1 are capable of bursting AML from the pre-existing clonal hematopoiesis context, but not directly from normal BM cells.16,17 On the other hand, we did not observe the occurrence of JAK2 V617F mutations in the AML group with myelodysplasia-related changes, suggesting that they are probably not involved in the MDS to AML clonal evolution. Indeed, JAK2 mutations have been previously associated with lower progression risk and longer survival in MDS.6,8,9,10,18
In summary, our preliminary observations suggest that mutations in the FLT3, NPM1, IDH1 and IDH2 genes can be potential biomarkers of disease progression in MDS patients. Studying the dynamics of these mutations in MDS and integrating this data with other approaches, such as cytology, karyotype and immunophenotype, may contribute to a more refined detection of disease progression in earlier stages, providing a broader window of opportunity for therapeutic intervention. However, further investigations with sequential sampling follow-ups during disease progression will be necessary to confirm current findings.
Author's contributionMatheus F Bezerra and Bruna R Larrazabal performed experiments, analyzed data and drafted the manuscript. Aleide S Lima, Mariana R Mello and Raphael F Pimentel performed experiments and analyzed medical files. Isabel Weinhäuser, Fernando F Costa, Kleber Y Fertrin and Aderson S Araújo analyzed medical files and reviewed the manuscript. Cíntia G Machado, Marcos A Bezerra and Antonio R Lucena-Araujo performed the conception and design of the study, reviewed the manuscript and gave the final approval of the version to be submitted.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by the Fundação de Amparo a Pesquisa do Estado de Pernambuco (FACEPE: Grant #0513-2.02/10) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: Grant #481904/2010-7).
We would like to thank to Dra. Ana Paula Freire Cavalcanti for her contribution in the analysis of the medical records.