The availability of multiple tyrosine kinase inhibitors (TKIs) for the management of chronic myeloid leukemia (CML) has created the debate on which one to use frontline.1,2 This debate would have been a straightforward one had there not been the huge cost differences between the first and second generation TKIs. The importance of early and deeper molecular responses on the risk of progression and blast crisis has been proven in the DASISION and ENESTnd trials leading to the approval of both dasatinib and nilotinib as first line TKIs along with imatinib in both the National Comprehensive Cancer Network (NCCN) and European Leukemia Net (ELN) guidelines.3–5 However, imatinib continues to be the most commonly used first line TKI for CML, especially in developing countries like India.
Patients with CML who are started on imatinib at diagnosis and fail to achieve the appropriate milestones as defined in the ELN guidelines are shifted to any one of the second generation TKIs and then continued on the same indefinitely until progression to blast crisis or allogeneic stem cell transplantation.6 Reintroduction of imatinib after achieving major molecular response (MMR) with a second generation TKI in a patient with primary imatinib failure indicative of a high risk disease would be theoretically incorrect and has not been reported in the literature. However, in this patient this option was adopted due to special circumstances with surprising results.
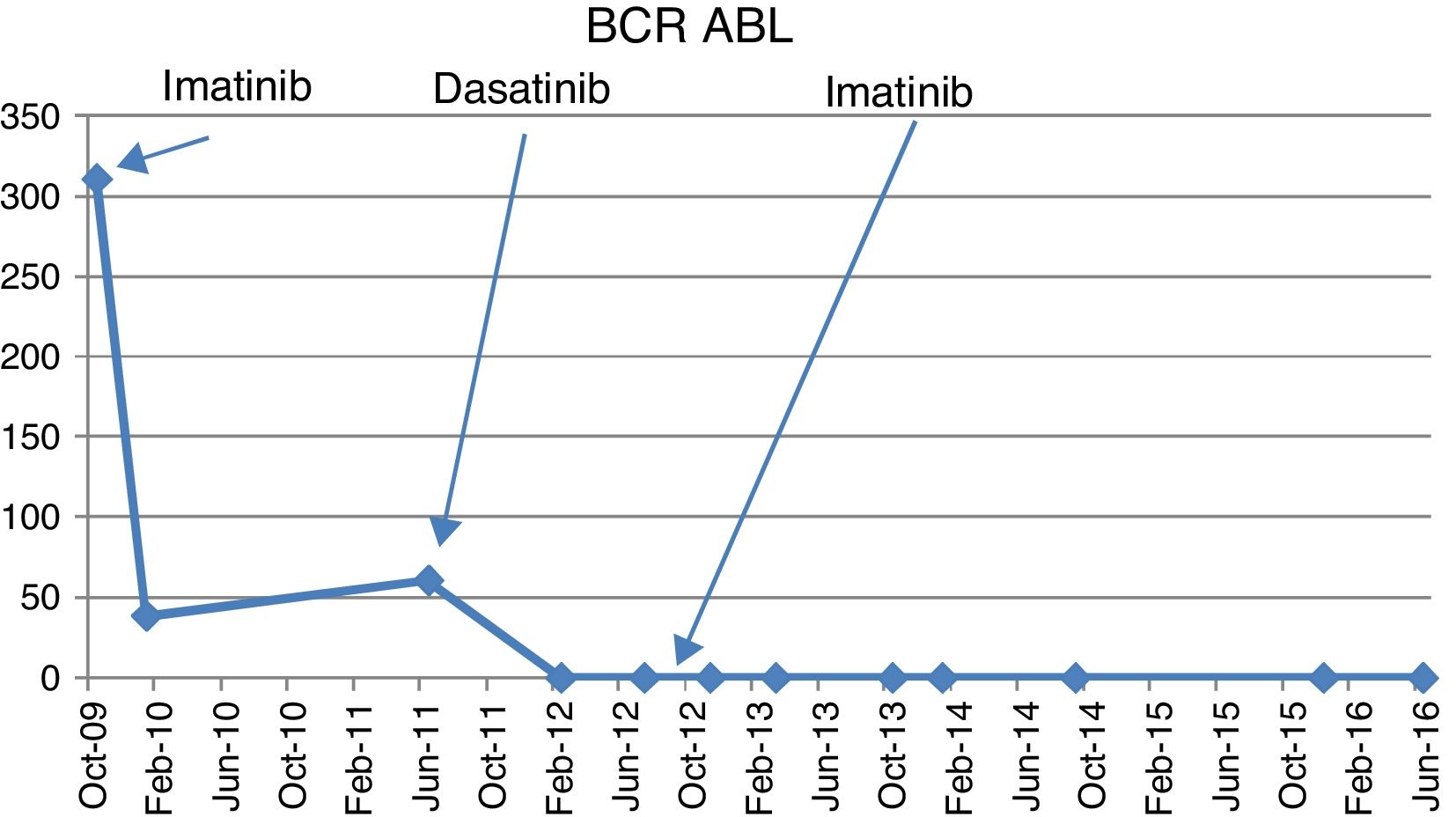
Case reportA 66-year-old male presented in September 2009 to a private practitioner with complaints of dyspepsia and was found to have mild leukocytosis with a total leukocyte count (TLC) of 14.8×103/μL. He was referred to our hematology center for evaluation. Clinically he had no positive findings except for the tip of his spleen being palpable. A peripheral blood smear showed a shift to the left with mild basophilia but no blasts. The leukocyte alkaline phosphate score was 10/58. A bone marrow examination showed marked granulocytic and megakaryocytic hyperplasia with panmyelosis and no increase in blasts. Karyotyping showed 20/20 metaphases positive for t(9;22). A quantitative real-time polymerase chain reaction (RQ-PCR) for the breakpoint cluster region-Abelson murine leukemia (BCR-ABL) was positive for p210 at 311%. He was started on imatinib (400mg OD) in October 2009. The quantitative RQ-PCR for BCR-ABL in January 2010 at three months was 38.56% but he achieved complete hematologic response (CHR). He was advised to attend monthly follow-ups. However, he followed up with his local practitioner and he was continued on imatinib (400mg OD) and serial RQ-PCR BCR-ABL studies were not carried out. He returned to our center in June 2011 when he continued to be asymptomatic and was in CHR but his RQ-PCR for BCR-ABL performed at this point was 60.71%. He claimed good compliance with imatinib treatment during this period, as was corroborated by the prescriptions of the peripheral hospital. A repeat bone marrow karyotype showed t(9;22) positive with no additional cytogenetic abnormalities while a tyrosine kinase domain (TKD) mutation analysis was negative. He was started on dasatinib (100mg OD) in August 2011. The repeat RQ-PCR for BCR-ABL in February 2012 was 0%. However, he was not tolerating dasatinib well with repeated episodes of loose stools, decreased appetite and abdominal discomfort. A repeat RQ-PCR for BCR-ABL in July 2012 at one year of dasatinib was 0%. Due to continued intolerance and refusal of the patient to take dasatinib and non-availability of nilotinib, the patient was restarted on imatinib (400mg OD) in August 2012. The patient was subsequently tested by RQ-PCR for BCR-ABL on a six-monthly basis [November 2012 (0.00%), March 2013 (0.069%), October 2013 (0.062%), January 2014 (0.008%), September 2014 (0.0024%), December 2015 (0.00%), and June 2016 (0.00%)] (Figure 1). Until 2014, the International Scale (IS) was not available for RQ-PCR in BCR-ABL monitoring at our institution. The reference control gene used was total ABL gene. Since 2015 onwards the IS scale of RQ-PCR for BCR-ABL was used for quantification. The patient has maintained ≥MMR for almost four years on imatinib post achievement of MMR with dasatinib and continues to be asymptomatic.
DiscussionThe concept of achieving deeper molecular responses with first generation TKIs for a sufficient period of time has been the basis for considering TKI withdrawal, looking for a possible cure in CML. This was defined as a 4-log reduction of BCR-ABL1 transcripts [molecular response (MR)4.0] for more than two years in the Stop Imatinib (STIM) trial as the criteria for imatinib withdrawal. However, the results have shown that 60% of patients had molecular relapse necessitating the reintroduction of TKIs. Hence, deep molecular response does not equate with a cure.7
This same concept was tried in a different setting in a few cases where there was initial imatinib failure due to TKD mutations but deep molecular responses were obtained with the 2nd generation TKI dasatinib. Dasatinib cessation was tried in these cases as well, with no relapse. The presence of BCR-ABL positive cells in the blood of a patient in a stable drug-free CMR appears to indicate that eradication of the leukemic clone is not a pre-requisite for the achievement of an operational cure of CML.8
Given the economics of 2nd generation TKIs in developing countries and the results of these two studies, theoretically one may consider the reintroduction of imatinib in patients who initially fail imatinib but do not have a TKD mutation and achieve deep molecular remission with dasatinib/nilotinib. This approach may be especially useful in the resource-limited setting of developing countries, if backed by adequate clinical data. However, it is important to note that restarting imatinib can only be considered in the context of initial deep molecular responses being achieved by a second generation TKI or if the BCR-ABL milestones are not adequately achieved (suboptimal responses) in the first year of therapy with imatinib and are subsequently achieved with second generation TKI and TKD mutation analysis shows no mutations.
In our case, there was primary imatinib failure/resistance after continued exposure for one and a half years which was followed by rapid achievement of MMR with dasatinib within six months. As no TKD mutations could be detected, other mechanisms of imatinib resistance might have played a role. However, the subsequent maintenance of MMR with imatinib is intriguing.
Imatinib, a phenylaminopyrimidine TKI specifically targets BCR-ABL1, KIT, and PDGFR kinases.9 Dasatinib on the other hand has a much wider spectrum by targeting >100 kinases including BCR-ABL1, PDGFR, c-KIT, SRC family, EPHA family, BTK, BMX, c-FMS, etc. Moreover, dasatinib targets an earlier progenitor population than imatinib and is likely more effective than imatinib within the stem cell niche.10 The mechanisms of imatinib resistance include BCR-ABL1 dependent and independent pathways. Pharmacokinetic considerations, intracellular uptake of imatinib, CML stem cell quiescence, clonal evolution and SRC overexpression are the major BCR-ABL independent mechanisms for resistance. BCR-ABL overexpression and TKD point mutations lead to BCR-ABL dependent resistance.9
The most plausible explanation of the phenomenon seen in this case appears to be the existence of a predominant additional subclone with dominant alternative tyrosine kinase as the driver for CML which was not affected by imatinib but was overcome by dasatinib. Overexpression of SRC kinase is a known mechanism of primary imatinib resistance and dasatinib has a significant inhibition of SRC kinases.9 Overexpression and/or activation of HCK and LYN has been implicated in CML progression to blast phase and imatinib resistance.11,12 In fact, LYN kinase has been shown to function as a regulator of imatinib sensitivity in CML, and it is found persistently activated in patients after failure of imatinib therapy who carry no BCR-ABL1 mutations.13 These observations indicate that in cells with high LYN expression, a combined approach targeting both BCR-ABL and LYN kinases may be necessary to overcome this form of imatinib resistance. This can be one of the explanations of the above phenomenon. Once a deep molecular remission was achieved by dasatinib leading to near elimination of the clone with dominant SRC kinase/LYN mediated proliferation, the subsequent use of imatinib was successful in maintaining the MMR. In addition, BCR-ABL directly interacts with multiple Src Family Kinases (SFKs) that subsequently switch the Abl kinase into an open, active conformation and phosphorylation of the SH2 and SH3 domains of BCR-ABL by the SFKs may increase the activity of the Abl kinase, thus influencing its susceptibility to imatinib.14 Hence, it can be speculated that there could also be cooperative tyrosine kinase activation amplifying each other which was disrupted by the sequential use of imatinib and dasatinib and subsequent imatinib reuse leading to MMR in this case.
BCR-ABL overexpression may contribute to BCR-ABL dependent resistance.6 At the onset, quantitative RQ-PCR identified BCR-ABL levels of 311% in our patient; this could be an indicator of BCR-ABL overexpression and contribute to initial imatinib failure. However, once it was suppressed by a more potent TKI, dasatinib, which has 325-fold greater activity against native Bcr-Abl compared to imatinib,15 the reintroduction of imatinib successfully maintained the MMR.
Polymutation is also well known in CML leading to TKI resistance.9 In one of the studies by Quintás-Cardama et al., 61 patients with CML after imatinib intolerance (n=10) or imatinib resistance (n=51) who received therapy with dasatinib were studied by DNA expansion of specific clones followed by DNA sequencing of at least ten clones. This study demonstrated the presence of 118 distinct mutations (77 previously not reported) involving 112 amino acids. More than 90% of patients harbored BCR-ABL kinase domain mutations prior to the start of dasatinib, and polymutants were detected in 57% of patients.16 The treatment of patients carrying polymutants may require the use of a combination of TKIs with activity against all single-point mutations contained in the compound mutation. However, in our case no TKD mutation was detected.
ConclusionIdentification of patients with CML who could benefit with short-term usage of 2nd generation TKIs, either at the onset or on imatinib failure, leading to deep molecular responses (≥MMR), may help in shifting them back to imatinib if these responses could be maintained by imatinib once the high-risk molecular character of the disease biology has been taken care of. This approach, if proven in larger studies, may be of considerable importance in the management of CML patients in resource-limited settings where the benefits of a 2nd generation TKI may be extended to a larger number of patients. Experience with the use of 2nd generation TKIs in resource-limited settings has made it clear that it is not feasible to implement Western guidelines on their usage in toto and rationalization is the need of the hour so that maximum benefits can be obtained from this wonderful class of drugs that has changed the landscape of CML management.
Ethical approvalInformed consent was obtained from all individual participants included in the study.
Conflicts of interestThe authors declare no conflicts of interest.