Due to the limited availability of organ donors and the growing number of patients awaiting orthotopic liver transplantation (OLT), non-ABO identical transplantation is often performed, in an attempt to lower the morbidity and mortality rates of transplant lists.1 Minor ABO incompatibility is described as the presence of naturally occurring ABO antibodies against the recipient red blood cells (RBCs) of the donor. Donor viable, immunocompetent lymphocytes present within the graft (known as passenger lymphocytes) are transferred and can produce antibodies against RBCs if they are stimulated shortly after transplant by recipient or transfused red cell antigens.2,3 Passenger lymphocyte syndrome (PLS) can also occur due to the transfer of lymphocytes that produce other anti-RBC antibodies.4,5
PLS is associated with different types of transplants. In solid organ transplants, the incidence of PLS is lowest in kidney, followed by liver and heart-lung transplants.6 PLS can also occur in allogeneic hematopoietic stem cell transplantation.7
Biochemical PLS, indicated by a derangement of the laboratory parameters of hemolysis, is relatively common in liver transplantation, affecting up to 37% of the patients undergoing minor ABO incompatible OLT.8 Therefore PLS is not an uncommon cause of anemia in non-ABO identical OLT, but often goes undiagnosed. The aim of this article is to describe two cases of PLS in liver transplantation and provide a literature review of this complication.
Case report 1A 57-year-old man with diabetes, hypertension and coronary heart disease was diagnosed with alcoholic liver cirrhosis in October 2013. He presented with severe and frequent episodes of hepatic encephalopathy and hemorrhagic events. OLT was performed using the piggyback technique in September 2014. His MELD score was 16. The donor was ABO group O+ and the recipient was A+. The patient received four A+ RBC units. Immunosuppression consisted of hydrocortisone, tacrolimus and mycophenolic acid. He was discharged from the hospital eight days after the transplantation, with hemoglobin (Hb) concentration of 8.52g/dL.
Four days later, he was readmitted to the hospital because of anemic syndrome. His exams showed: Hb of 5.03g/dL, positive direct antiglobulin test (DAT – IgG1+, C3d2+), reticulocytosis of 393×103/μL, lactate dehydrogenase (LDH) of 544U/L (normal range<460U/L) and total bilirubin of 1.24mg/dL. Laboratory coagulation values (platelet count, partial thromboplastin time, prothrombin time) were all within the normal ranges. Ultrasound screening was performed and there was no evidence of intra-abdominal bleeding. An ABO discrepancy was found and anti-A1 antibodies were detected in the eluate and in the serum, with titration of 4+. The patient received four leukoreduced RBC units (two of A2 blood type and two of O blood type) according to the protocol for transfusion of liver transplant recipients in our institution and his clinical condition improved. He was discharged five days later with stable Hb and asymptomatic.
Case report 2A 13-year-old girl was diagnosed with acute liver failure, Child C(12) with PELD 32 in August 2015. She underwent OLT with no excessive bleeding or other complications. The recipient blood type was B+ and donor blood type was O+. Immunosuppression consisted of hydrocortisone, tacrolimus and mycophenolic acid. The patient was discharged from the hospital eight days after surgery. On Day 11 post-transplant her Hb concentration was 9.54g/dL.
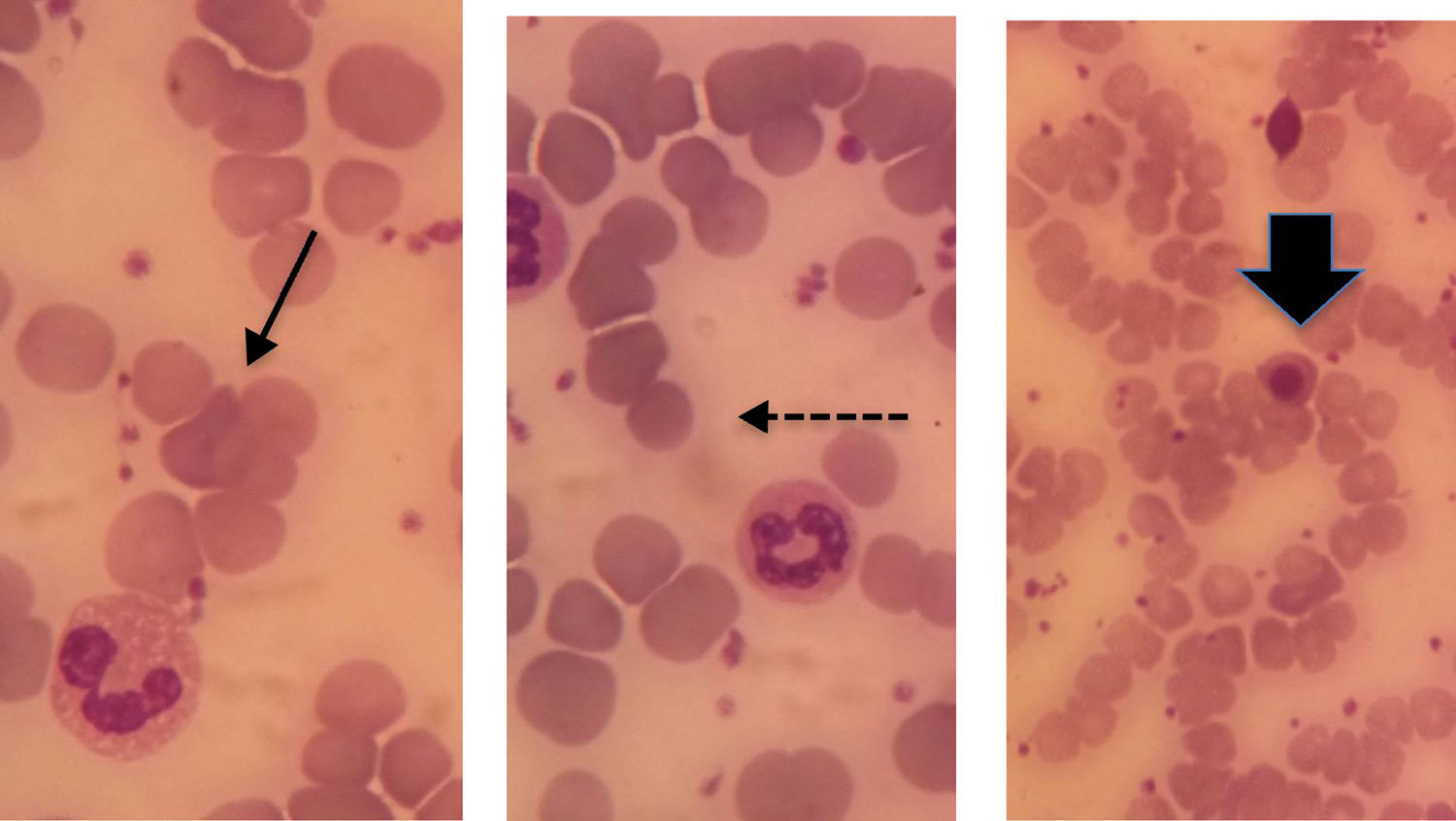
On day 13, she was readmitted because of a sudden decrease in Hb concentration. Her exams showed: Hb of 5.8g/dL, positive DAT (IgG2+), reticulocytosis of 240.19×103/μl (normal value <89.0×103/μL), LDH of 798U/L (normal value<460U/L), total bilirubin of 2.51mg/dL, indirect bilirubin of 1.55mg/dL with no evidence of bleeding in abdominal ultrasound. Spherocytes and erythroblasts were noted on the peripheral smear (Figure 1). An ABO discrepancy was found and anti-B antibodies were detected in serum and eluate. The patient received folic acid and hydration with improvement of clinical condition and hemoglobin levels after three days. She was discharged after seven days. The post-transplant reevaluation on Day 26 showed an Hb concentration of 11.1g/dL.
DiscussionLiver transplantation can be associated with many hematological abnormalities.2 Graft-versus-host disease, post-transplant lymphoproliferative malignancies, thrombotic microangiopathy, hemophagocytic syndrome induced by infections and PLS are among the hematological complications of liver transplantation.2 Since anemia can occur in more than 50% of liver transplant recipients,9 the differential diagnosis of anemia and jaundice also includes inferior vena cava and hepatic vein thrombosis, portal vein thrombosis, hepatic artery thrombosis and stenosis, biliary complications, and infections or sepsis.10
PLS is a well-known syndrome of immune hemolysis following allogeneic hematopoietic stem cell11 or solid organ transplantations, such as kidney,12,13 liver4 and heart-lung transplants.4,14 Patients of A, B or AB blood groups may receive organs from ABO-compatible, but non-identical donors.15 These minor ABO incompatible transplantations occur more frequently in: (a) the use of allografts from live donors, (b) acute liver failure, (c) urgent re-transplants and (d) AB blood group patients. PLS is more frequent with donor O and recipient A.14 Both of our patients had PLS due to minor ABO incompatibility (donors O and recipients A and B).
The frequency of PLS after minor ABO incompatibility organ transplantation depends on the lymphoid mass transplanted14 with lymphocytes accounting for almost 4% of the liver mass.16 The incidence of PLS is about 13.5%13 in kidney and 70% in heart and in lung transplants.17 Ramsey et al. described an incidence of 37% of PLS in liver transplantation in a retrospective analysis of 1000 patients.8 Another recent retrospective study at a transplant center in Spain detected 12 PLS in a total of 1217 OLT.18 Ten patients of 56 OLT with minor ABO incompatibility developed PLS (17.9%) and two patients of 147 cases with minor Rh incompatibility developed the syndrome (1.40%).18 On the other hand, in a prospective analysis of eleven ABO or RhD mismatched liver transplantations, ElAnsary et al. found only two PLS with antibodies directed against ABO or RhD in the serum or eluate.19 Although, a positive DAT was encountered in six of the eleven patients.19
The PLS may be considered a type of graft-versus-host disease, according to Audet et al.,3 where donor immunocompetent memory B lymphocytes escape from immune surveillance of the immunosuppressed recipient and are stimulated to produce antibodies directed against RBC antigens (or of transfused RBC), causing hemolysis. The importance of donor-derived memory B lymphocytes within the transplanted organ is highlighted by two case series,4,20 as hemolysis was observed in more than one organ recipient from the same donor.
The PLS usually has a sudden onset14 of between four days4 and three weeks after transplantation,2 and the clinical presentation of PLS ranges from mild and compensated hemolysis to severe and possibly fatal anemia with kidney failure.18,21 The patient of Case Report 1 was diagnosed with PLS on the 12th day and of Case Report 2, on the 15th day after OLT. They had significant decreases in Hb concentration with altered hemolysis screen and no evidence of bleeding to justify the anemia.
Besides a decrease in Hb concentration, laboratory abnormalities in PLS include alterations in hemolysis markers such as increased indirect bilirubin and LDH, decreased haptoglobin, in addition to the presence of a positive DAT.14,21 Both patients described herein had anemic symptoms with very mild jaundice, hardly noticed at physical examination, although indirect bilirubin was increased. They also had the other abnormalities found in PLS such as reticulocytosis, elevated LDH and positive DAT (one IgG and C3d; the other IgG).
The presence of an antibody with a known specificity against a host RBC antigen in the serum and/or in the eluate is necessary for diagnosis.6,22 Both our patients had ABO discrepancies in blood tests and there were antibodies against ABO antigens in the eluate (anti-A1 in the first case and anti-B in the second case). Although most cases of PLS are due to ABO incompatibility, other antibodies against red cell antigens such as Rh,23 Kell,4,20,24 Kidd5 and Duffy20 have been described.
The disease is often self-limiting, usually resolving within three months, because the passenger lymphocytes do not engraft and there is a finite time during which the viable lymphocytes can proliferate.2,6 However, Fung et al. described a severe case of PLS after OLT that only resolved with splenectomy almost one year after the diagnosis.23
Treatment is supportive and consists of simple transfusions with blood products of donor blood group and, in severe cases, erythrocytapheresis can be performed23 to remove incompatible recipient-origin red blood cells and, consequently, the amount of the target antigen.2 Rituximab has also been used with reported success.25 Steroids have not been shown to be of benefit in treating hemolysis in this setting.21 Both our patients improved within a few days with supportive therapy and the maintenance of the same dose of prednisone they were already using. The patient in Case Report 1 was elderly with coronary heart disease and his Hb decreased to 5.03g/dL so he received four RBC units matched to the liver donor. The patient of Case Report 2 did not receive RBC transfusions.
As anemia is a frequent finding in patients undergoing OLT, the transplantation team must always consider PLS in patients with abrupt decreases of Hb concentrations and no sign of bleeding, particularly if the recipient received an organ from a minor ABO incompatibility donor, or if the donor was tested positive in RBC antibody screening. Furthermore, these two cases illustrate well that PLS is usually a self-limiting condition and the change in the immunosuppressive scheme is not always required, since it may increase the risk of infective complications that are already common in transplanted patients. Perhaps a more aggressive treatment is only justified in hemolysis with renal repercussion, in the patients where it is not possible to maintain safe levels of hemoglobin only with transfusions or in those with hemolysis persisting for periods longer than two weeks, during which time PLS usually resolves.