Mutations affecting genes involved in oxidative and signaling pathways may be associated with kidney disease in sickle cell anemia. We determined the allele and genotype frequencies of some polymorphisms in the promoter regions of the Heme Oxygenase-1 (HMOX1) [rs2071746 (A>T) and (GT)n repeats, short (S) and long (L) alleles] and Bone Morphogenetic Protein Receptor type-1B (BMPR1B) [rs17022863 (A>G), rs4331783 (A>G) and rs1470409 (A>G)] genes in 75 adult patients with sickle cell anemia and 160 healthy controls and investigated whether these polymorphisms may influence the estimated glomerular filtration rate for the patients.
MethodsThe single nucleotide polymorphisms were genotyped using the TaqMan assays, the HMOX1(GT)n repeats were determined by polymerase chain reaction fragment size analysis and the estimated glomerular filtration rate was calculated by the Modification of Diet in Renal Disease formula.
ResultsRegarding the HMOX1rs2071746, the estimated glomerular filtration rate median was significantly higher in TT patients (p=0.019), including when TT was compared with AT+AA (p=0.009); for the (GT)n repeats, the estimated glomerular filtration rate medians of SS, SL and LL significantly differed (p=0.009), being the LL estimated glomerular filtration rate median significantly higher, when compared with the LS+SS (p=0.005). These results suggest that both the homozygotes, TT for rs2071746 and LL for (GT)n repeats, lead to a higher risk of developing renal complications. Concerning the BMPR1B, the frequencies of GG for rs17022863 and AA for rs4331783 were significantly higher in patients than in controls (p=0.002 and p=0.008, respectively), however no association with estimated glomerular filtration rate was found.
ConclusionThese results contribute to a better understanding of the genetic factors related to the development of nephropathy in sickle cell anemia patients.
Sickle cell anemia (SCA) is a chronic and severe hemolytic anemia associated with endothelial dysfunction, vaso-occlusion and inflammation. It is caused by a homozygous inheritance of a single point mutation (GAG>GTG) at the 7th codon of the β globin gene (according to the Human Genome Variation Society nomenclature), on 11p15.5, resulting in the replacement of glutamic acid for valine at the 7th position of the β globin chain and leading to the formation of hemoglobin S (HbS) (HBB:c.20A>T; p.E7V). Deoxy-HbS polymerizes inside the erythrocytes, resulting in the formation of sickled red blood cells, related to hemolysis and vaso-occlusive events, organ damage and a wide range of clinical manifestations.1,2
One of the most important clinical complications in SCA is kidney disease, responsible for 15–18% of the mortality rate in adult patients.1,2 In the early stages, it includes glomerular hyperfiltration, glomerular enlargement, and hematuria,3–5 progressing to chronic kidney disease (CKD).6 Hemolysis seems to be related to the pathogenesis of renal disease in SCA.7–9 Chronic hemolysis results in high renal plasma flow, which can lead to endothelial dysfunction, causing hemodynamic changes that result in renal functional and structural abnormalities and, consequently, an increase in the glomerular filtration rate (GFR).4,5,9 Genetic variants of genes related to oxidative and signaling pathways, such as the Heme Oxygenase-1 (HMOX1) and Bone Morphogenetic Protein Receptor Type 1B (BMPR1B) genes, respectively, have been associated with some of the clinical complications in SCA, such as stroke, osteonecrosis and acute chest pain.10–12 Studies have revealed that the release of free heme in these patients triggers the formation of reactive oxygen species (ROS) and oxidative stress, induces HMOX1 activity and increases the conversion of oxidized angiotensinogen to angiotensin II, which mediates the generation of signaling molecules, capable of inducing systemic responses leading to renal damage.13–16
The HMOX1 is the rate-limiting enzyme that degrades heme through oxidation to yield equimolar quantities of biliverdin, carbon monoxide (CO) and free iron (Fe).17 A severe, irreversible renal failure with 100% mortality was found in mice deficient in Hmox1 (−/−) in contrast with non-deficient Hmox1 (+/+) mice, that showed mild, reversible renal injury and 0% mortality.18 The HMOX1 gene has two promoter variants that have been widely studied, a −413 A>T single nucleotide polymorphism (SNP) (rs2071746) and a (GT)n microsatellite polymorphism.19 While the first has been associated with the fetal hemoglobin levels and clinical phenotypes of SCA patients,20,21 in the latter, short repeats (n≤25 or n<27, according to the cutoff of different studies) have been associated with higher gene expression levels than the long (GT)n repeats (n≥25 or n≥27).19
The BMPR1B gene encodes a member of the bone morphogenetic protein (BMP) receptor family of transmembrane serine/threonine kinases. The ligands of this receptor are bone morphogenetic proteins (BMPs), which are members of the transforming growth factor beta (TGF-beta) superfamily. The BMPs are involved in endochondral bone formation and embryogenesis. These proteins transduce their signals through the formation of heteromeric complexes of two different types of serine (threonine) kinase receptors: type I receptors of approximately 50–55kD and type II receptors of approximately 70–80kD.22 Patel et al. observed that some SNPs in the BMPR1B gene are associated with nephropathy in Type 1 diabetes;23 Nolan et al., studying SCA patients, found that the SNP rs17022863 and the combination of SNPs rs17022863, rs4331783 and rs1470409 in haplotypes are significantly associated with the estimated GFR (eGFR).24
In Brazil, the prevalence of the βs allele is high, varying from 1.2% to 10.9%, depending on the region of the country.2 To our knowledge, there are no studies evaluating the influence of polymorphisms in the HMOX1 and BMPR1B genes on the renal function in Brazilian SCA patients. Thus, the aim of this study was to determine and compare the allelic and genotypic frequencies of the SNP rs2071746 and (GT)n repeats in the HMOX1 gene and the SNPs rs17022863, rs4331783 and rs1470409 in the BMPR1B gene between Brazilian adult SCA patients and healthy controls and to investigate whether these polymorphisms may influence the eGFR of these patients.
MethodsThe study comprised 75 SCA patients (HbSS, 28.3±8.2 years old, 54.7% males) followed at the Hematology and Hemotherapy Center of Pernambuco (HEMOPE), in Recife, Pernambuco in northeastern Brazil. Inclusion criteria for the study required that patients with SCA were in steady state and without hydroxyurea treatment for at least three months. Additionally, patients who had received blood transfusion in the previous three months or those who already had had a renal failure diagnosis (eGFR<60mL/min/1.73m2) were excluded from the study. The control group was composed of 160 healthy adults (blood donors) (HbAA, 40±10.1 years old, 78% males) from the same Brazilian region, with ethnic characteristics similar to those of the patients. Further information on the biodata was obtained by interview and from patient medical records. The local ethics committee approved this study, and informed consent was obtained from all participants, according to the Helsinki Declaration.
The genomic DNA from all patients and controls was extracted from peripheral blood, using commercially available kits (GFX™ Genomic Blood DNA Purification Kit; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), according to the manufacturer's instructions. The SNPs were genotyped by the TaqMan® SNP Genotyping Assay in the Step One Plus Real-Time Polymerase Chain Reaction (PCR) system, according to the manufacturer protocol (Applied Biosystems, Foster city, CA, USA). The (GT)n repeats were identified by capillary electrophoresis on the ABI3500 Genetic Analyzer, using the Gene Mapper version 4.1 Software (both Applied Biosystems, Carlsbad, CA, USA). They were classified according to Bean et al., as short (S) with ≤25 repeats and long (L) with ˃25 repeats.13 Of note, these genetic variants were selected based on their functional relevance in protein levels16,25 or in an eGFR context.24
The eGFR was determined in the SCA patients by using the simplified prediction equation MDRD (Modification Diet in Renal Disease): eGFR (mL/min/1.73m2)=175×(serum creatinine mg/dL)−1.154×(age)−0.203×0.742 (if female).7 For statistical comparisons, renal failure was defined as eGFR<60mL/min/1.73m2 and renal hyperfiltration as eGFR>130mL/min/1.73m2 and >140mL/min/1.73m2 for women and men, respectively.7
The Statistical Analysis System (SAS) for Windows version 9.4 (SAS Institute Inc., Cary, USA) was used for the analysis. Allelic and genotypic frequencies were determined in both patients and controls. The Hardy-Weinberg equilibrium (HWE) was verified by the X2 test. Age and gender were adjusted using regression logistics. The Fisher's exact test or Chi-square test, as appropriate, was used to compare categorical variables. The one-way ANOVA or Mann–Whitney test, as appropriate, was used to analyze the eGFR data exclusively on patients with SCA. The p-values <0.05 were considered statistically significant.
ResultsClinical data of patientsOut of the 75 SCA patients, 40 patients (53.3%) had vaso-occlusive crises which required hospitalization/year in the previous year. Other complications reported were gallstones (n=47, 62.7%), pneumonia (n=33, 44.0%), chronic leg ulcers (n=17, 22.7%), aseptic necrosis (n=11, 14.7%), and stroke (n=4, 5.3%). Of the 44 male SCA patients, 7 (15.9%) had experienced priapism in the past.
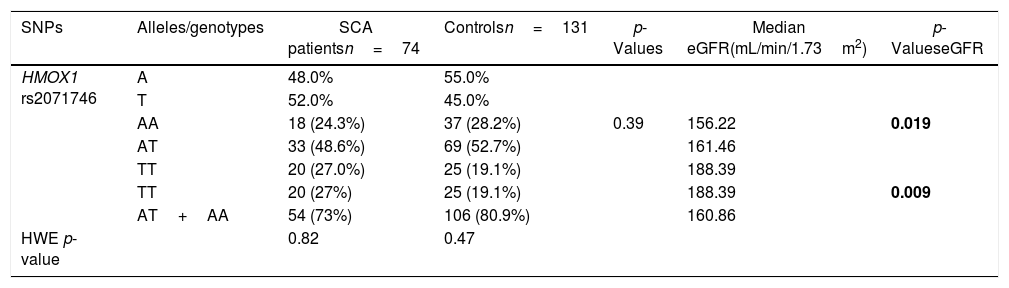
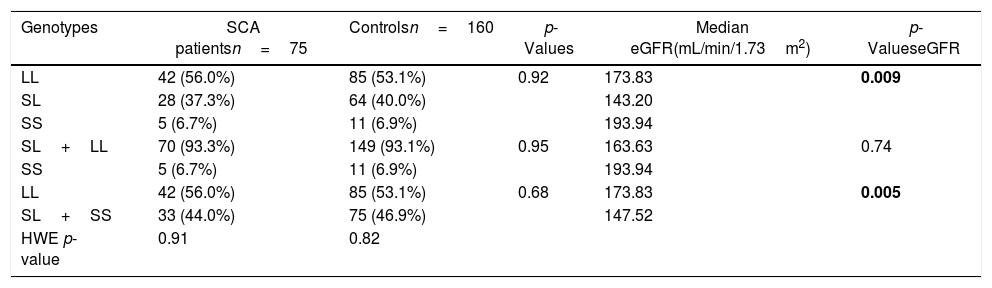
Allelic and genotypic distributions of HMOX1 promoter polymorphismsThe allelic and genotypic frequencies for HMOX1 rs2071746 and HMOX1 (GT)n repeats are presented in Tables 1 and 2, respectively. The HMOX1 rs2071746 genotype frequencies were 24.3% (AA), 48.6% (AT) and 27.0% (TT) in the SCA patient group and 28.2% (AA), 52.7% (AT), 19.1% (TT) in the control group. The rs2071746 allele frequencies were 48% (A) and 52% (T) in patients and 55% (A) and 45% (T) in controls. The distribution of the genotypes was in accordance with the Hardy-Weinberg equilibrium, both in patient and control groups (pSCA=0.82 and pcontrol=0.47, respectively). Regarding the (GT)n repeats in HMOX1, genotype frequencies were 56.0% (LL), 37.3% (SL) and 6.7% (SS) in the SCA patient group and 53.1% (LL), 40.0% (SL) and 6.9% (SS) in the control group. All genotype frequencies determined were in Hardy-Weinberg equilibrium (pSCA=0.91; pcontrol=0.82, respectively). For HMOX1 rs2071746 and (GT)n repeats, no significant difference was observed in the distribution of different genotypes and alleles in both patient and control groups.
Allele and genotype frequencies for the rs2071746 in the HMOX1 gene.
| SNPs | Alleles/genotypes | SCA patientsn=74 | Controlsn=131 | p-Values | Median eGFR(mL/min/1.73m2) | p-ValueseGFR |
|---|---|---|---|---|---|---|
| HMOX1 rs2071746 | A | 48.0% | 55.0% | |||
| T | 52.0% | 45.0% | ||||
| AA | 18 (24.3%) | 37 (28.2%) | 0.39 | 156.22 | 0.019 | |
| AT | 33 (48.6%) | 69 (52.7%) | 161.46 | |||
| TT | 20 (27.0%) | 25 (19.1%) | 188.39 | |||
| TT | 20 (27%) | 25 (19.1%) | 188.39 | 0.009 | ||
| AT+AA | 54 (73%) | 106 (80.9%) | 160.86 | |||
| HWE p-value | 0.82 | 0.47 |
HWE: Hardy-Weinberg equilibrium; n: number; SNP: single nucleotide polymorphism; eGFR: estimated glomerular filtration rate.
Distribution of genotype frequencies of the (GT)n repeats in the HMOX1 gene.
| Genotypes | SCA patientsn=75 | Controlsn=160 | p-Values | Median eGFR(mL/min/1.73m2) | p-ValueseGFR |
|---|---|---|---|---|---|
| LL | 42 (56.0%) | 85 (53.1%) | 0.92 | 173.83 | 0.009 |
| SL | 28 (37.3%) | 64 (40.0%) | 143.20 | ||
| SS | 5 (6.7%) | 11 (6.9%) | 193.94 | ||
| SL+LL | 70 (93.3%) | 149 (93.1%) | 0.95 | 163.63 | 0.74 |
| SS | 5 (6.7%) | 11 (6.9%) | 193.94 | ||
| LL | 42 (56.0%) | 85 (53.1%) | 0.68 | 173.83 | 0.005 |
| SL+SS | 33 (44.0%) | 75 (46.9%) | 147.52 | ||
| HWE p-value | 0.91 | 0.82 |
HWE: Hardy-Weinberg equilibrium; n: number; L: long; S: short; eGFR: estimated glomerular filtration rate.
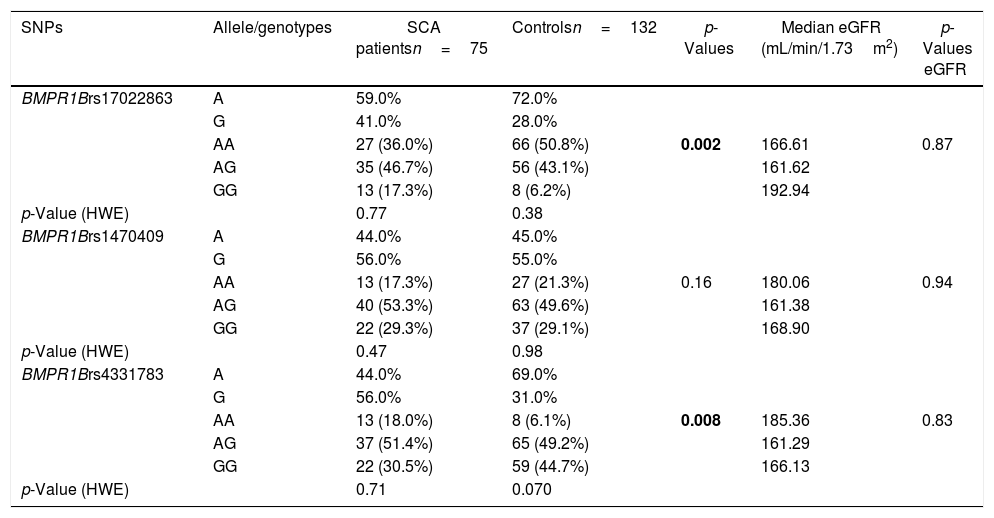
The allele and genotype frequencies for the rs17022863, rs4331783 and rs1470409 in the BMPR1B gene in SCA patients and controls are presented in Table 3.
The eGFR in patients with SCA was associated with both HMOX1 rs2071746 and (GT)n repeats polymorphisms. For the HMOX1 rs2071746, the median eGFR was significantly higher in patients with the TT genotype (TT vs. AA: 188.39 and 156.22mL/min/1.73m2, respectively, p=0.02; TT vs. (AT+AA): 188.39 and 160.86mL/min/1.73m2, respectively, p=0.009). Similarly, the median eGFR significantly differed according to HMOX1 (GT)n repeats genotypes (p=0.009), with a significantly higher median eGFR in the LL genotype, when compared with the LS+SS genotypes [LL vs. (LS+SS): 147.52 and 173.83mL/min/1.73m2, respectively, p=0.005].
Allelic and genotypic distributions of BMPR1B polymorphismsThe allelic and genotypic frequencies for rs17022863, rs4331783 and rs1470409 in the BMPR1B gene are presented in Table 3. The BMPR1B rs17022863 genotyping results were 36.0% (AA), 46.7% (AG) and 17.3% (GG) in the SCA patient group and 50.8% (AA), 43.1% (AG), 6.2% (GG) in the control group, with allele frequencies of 59.0% (A) and 41.0% (G) and 72.0% (A) and 28.0% (G), respectively. The distribution of the genotypes was in accordance with the Hardy-Weinberg equilibrium, both in patient and control groups (pSCA=0.77; pcontrol=0.38, respectively). The frequency of genotype AA for rs17022863 was significantly lower in patient than in control group (p=0.002). For BMPR1B rs1470409, the genotyping results were 17.3% (AA), 53.3% (AG) and 29.3% (GG) in the SCA patient group and 21.3% (AA), 49.6% (AG) and 29.1% (GG) in the control group. The rs1470409 allele frequencies for the patient and control groups were 44.0% (A) and 56.0% (G) and 45.0% (A) and 55.0% (G), respectively. The genotyping results were in Hardy-Weinberg equilibrium in both patients and controls (pSCA=0.47; pcontrol=0.98, respectively). There was no significant difference in the distribution of the genotypes and alleles in both patient and control groups. Concerning the BMPR1B rs4331783, the genotype frequencies were 18.0% (AA), 51.4% (AG) and 30.5% (GG) in the SCA patient group and 6.1% (AA), 49.2% (AG) and 44.7% (GG) in the control group. The rs4331783 allele frequencies for the patient and control groups were 44.0% (A) and 56.0% (G) and 69.0% (A) and 31.0% (G), respectively. However, the distribution of these genotypes in the control group was not in accordance with the Hardy-Weinberg equilibrium (pSCA=0.71; pcontrol=0.07). None of the analyzed BMPR1B polymorphisms (rs17022863, rs1470409 and rs4331783) were associated with eGFR (p=0.87, p=0.94 and p=0.83).
Allele and genotype frequencies for the rs17022863, rs4331783 and rs1470409 in the BMPR1B gene.
| SNPs | Allele/genotypes | SCA patientsn=75 | Controlsn=132 | p-Values | Median eGFR (mL/min/1.73m2) | p-Values eGFR |
|---|---|---|---|---|---|---|
| BMPR1Brs17022863 | A | 59.0% | 72.0% | |||
| G | 41.0% | 28.0% | ||||
| AA | 27 (36.0%) | 66 (50.8%) | 0.002 | 166.61 | 0.87 | |
| AG | 35 (46.7%) | 56 (43.1%) | 161.62 | |||
| GG | 13 (17.3%) | 8 (6.2%) | 192.94 | |||
| p-Value (HWE) | 0.77 | 0.38 | ||||
| BMPR1Brs1470409 | A | 44.0% | 45.0% | |||
| G | 56.0% | 55.0% | ||||
| AA | 13 (17.3%) | 27 (21.3%) | 0.16 | 180.06 | 0.94 | |
| AG | 40 (53.3%) | 63 (49.6%) | 161.38 | |||
| GG | 22 (29.3%) | 37 (29.1%) | 168.90 | |||
| p-Value (HWE) | 0.47 | 0.98 | ||||
| BMPR1Brs4331783 | A | 44.0% | 69.0% | |||
| G | 56.0% | 31.0% | ||||
| AA | 13 (18.0%) | 8 (6.1%) | 0.008 | 185.36 | 0.83 | |
| AG | 37 (51.4%) | 65 (49.2%) | 161.29 | |||
| GG | 22 (30.5%) | 59 (44.7%) | 166.13 | |||
| p-Value (HWE) | 0.71 | 0.070 |
HWE: Hardy-Weinberg equilibrium; n: number; SNP: single nucleotide polymorphism; eGFR: estimated glomerular filtration rate.
The HMOX1 enzymes have been known for their antioxidant and anti-inflammatory activities.26–28 In SCA, these enzymes are particularly important to protect patients from the damaging effects of excess heme released by high rates of chronic intravascular hemolysis.28 A recent study with SCA mice has shown that induction of the HMOX1 protein significantly inhibits inflammatory markers and vaso-occlusion.29 We determined the allelic and genotypic frequencies of two common polymorphisms in the promoter region of the HMOX1 gene in Brazilian adult SCA patients and compared them with those found in a control group. The frequencies were all in HWE and did not significantly differ between patients and controls. Additionally, we also investigated whether the different genotypes related to these two polymorphisms could influence the eGFR of our patients. Regarding rs2071746, we observed that the median eGFR was significantly higher in patients with the TT genotype (p=0.019), including when TT was compared with AT+AA (p=0.009). This finding agrees with previous studies, which found the association between the T allele and the reduction of the HMOX1 gene expression13,30 and suggests that patients with the TT genotype are at higher risk of developing renal complications. In relation to the (GT)n repeats, the median eGFR of the three genotypes (SS, SL and LL) significantly differed (p=0.009); when LL was compared with LS+SS, the LL median eGFR was significantly higher (p=0.005). This result also suggests that patients with the LL genotype may have a higher risk of renal disease progression, supporting the concept that long alleles are associated with decreased HMOX1 activity, as found by Chen et al. in patients with coronary artery disease.31
The BMPR1B gene plays an important role in cell proliferation, inflammatory response and tissue repair. In our patients and controls, the allele and genotype frequencies of the SNPs rs17022863, rs4331783 and rs14070409 were all in HWE; the frequencies for two SNPs, rs17022863 and rs4331783, were statistically different (p=0.002 and 0.008, respectively), in patient and control groups. This may be under selective pressure in patients with SCA. However, in contrast to Nolan et al.,24 there was no significant association between the genotypes and eGFR. This may be because of our relatively small sample size or differences in the ethnic composition of the populations analyzed.
To the best of our knowledge, this is the first study determining the allele and genotype frequencies of common polymorphisms in the HMOX1 and BMPR1B genes and investigating their association with eGFR in Brazilian SCA patients. This finding may contribute to a better understanding of the genetic factors related to the development of renal complications in SCA and provide additional support for the role of the HMOX1 gene in SCA nephropathy.
Limitations of the study: These findings should be interpreted with caution due to the sample size analyzed, the absence of micro- or macroalbuminuria and urinary protein excretion data and the need for validation in other cohorts.
FundingThis study was financially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Grant No. 2014/00984-3), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq) and Fundo de Apoio ao Ensino, Pesquisa e Extensão-FAEPEX/UNICAMP (Brazil).
Conflicts of interestThe authors declare no conflicts of interest.