Stroke is a serious complication of sickle cell anemia (SCA). The transcranial Doppler (TCD) is the risk-screening tool for ischemic strokes. The objective of the study was to describe the clinical progression of children with SCA who presented with high risk for stroke by TCD or relevant changes by magnetic resonance angiography (MRA) and underwent the regular transfusion program (RTP) and/or hydroxyurea (HU) treatment between 2007 and 2018.
MethodThis was a neonatal retrospective/prospective cohort study with children born between 1999 and 2014 with the homozygotic form (HbSS) or Sβ0-thalassemia who underwent TCD at least once.
ResultsOf the 718 children screened during this period, 675 had HbSS and 43 Sβ0-thalassemia. In 54 children (7.5%), all with HbSS, a high-risk TCD (n = 45) or, when the TCD was inconclusive, an MRA with cerebral vasculopathy (n = 9) was used for detection. Of these, 51 started the RTP and the families of three refused treatment. Of the 43 children with a high-risk TCD who initiated the RTP, 29 (67.4%) reverted to low risk. In 18 of them (62%), HU was started at the maximum tolerated dose (MTD) before transfusion discontinuation. None of these 29 patients had a stroke. Eight children (18.6%) maintained a high-risk TCD, even using the RTP/HU and two had a stroke.
ConclusionsThe TCD was confirmed as a viable tool for tracking patients with a risk for stroke. The RTP was effective in preventing the primary event. New strategies are necessary to prevent stroke using HU and new drugs, in addition to bone marrow transplantation.
Sickle cell anemia (SCA) is the most severe form of sickle cell disease (SCD). This subtype presents homozygosis of the βs alleles in the gene responsible for hemoglobin (Hb) chain β synthesis, resulting in HbS formation. Hb polymerization occurs under low oxygenation conditions, leading to structural red blood cell change. Successive deoxygenation events result in irreversible sickling, causing hemolysis, vessel occlusion, endothelial inflammatory reaction, ischemia and tissue reperfusion injury. Consequently, acute painful episodes, stroke, acute chest syndrome (ACS), acute splenic sequestration (ASS), priapism and other symptoms are observed.1
SCD has a high prevalence worldwide, being a public health problem in Brazil. In Minas Gerais, Brazil, neonatal hemoglobinopathy screening was implemented in March 1998. According to the data from the Diagnostic Support Action and Research Center (NUPAD), the homozygotic form (HbSS) has an incidence of 1:2400 live births. The sickle cell trait is present in 1:33 newborns. Screening, follow-up and treatment are provided by the Brazilian Public Health System (SUS).2
Cerebrovascular complications, including changes detected by transcranial Doppler (TCD) ultrasound, silent infarction (SI), transient ischemic attack (TIA) and clinical stroke are frequent and affect almost half of the patients aged up to 14 years.3
The Belo Horizonte Blood Center (HBH), affiliated with SUS, has registered stroke rates that corroborate the international literature. The incidence was 0.77/100 patient-years, with a cumulative clinical stroke probability of 8.3% at 10 years of age in a smaller cohort than the present one.4,5
Stroke presents as a sudden onset of acute neurological deficit and rapid progression resulting from new ischemic or hemorrhagic lesions identified by brain computed tomography (CT) and magnetic resonance imaging (MRI), causing motor and/or sensory focal neurological signs and symptoms lasting more than 24 h.6
The SCD stroke pathophysiology is still poorly understood. Although several predictors are known, basal hematological parameters, such as low basal hemoglobin level (< 7 g/dL) and high leukocyte and reticulocyte counts have been considered important predictors. Situations, such as hypoxemia, history of bacterial meningitis, prior TIA and SI detected with magnetic resonance imaging (MRI) and systolic arterial hypertension are associated with a higher risk of stroke. Additionally, acute events, such as recurrent or recent ACS and medullary aplasia by erythrovirus B19, may predispose to stroke.7-9
There is consensus in the literature that abnormal TCD (v.g., high-risk classification for stroke) in children indicates a chronic transfusion regimen to prevent the first episode, i.e., primary prevention using the regular transfusion program (RTP).10,11 However, the use of RTP after an abnormal TCD eventually treats about 40% of children who would not progress to clinical stroke, exposing them to repeated transfusions, which are not risk-free.12
TCD screening, combined with therapeutic intensification, whether isolated or combined RTP, bone marrow transplant and hydroxyurea (HU) therapy, reduced the risk of stroke in individuals under 18 years of age from 11% to 1.9%.3 Another study13 similarly showed that the incidence of stroke was reduced from 0.67 to 0.06 per 100 patient-years after the introduction of TCD.
TCD screening has been provided since 2007 to all children aged ≥ 2 years with SCA and Sβ0-thalassemia followed up at the HBH. It is a feasible, low-cost tool that is well tolerated by children. It has a sensitivity and specificity similar to those of cerebral angiography, which, although considered the gold standard test, is more expensive, presents risk of complications and requires hematological preparation of the patient.14
The objective of the study was to describe the clinical progression of children with SCA, who presented with a high risk for stroke by TCD or relevant changes detected by magnetic resonance angiography (MRA), and underwent the regular transfusion program (RTP), with or without hydroxyurea (HU) treatment, between 2007 and 2018.
Material and methodsA cohort study, part retrospective, part prospective, included children with SCD born between 1999 and 2014, with HbSS or HbSβ0-thalassemia diagnosed in the Neonatal Screening Program and followed up by HBH, in Belo Horizonte, Minas Gerais, Brazil. This program covers all cities in Minas Gerais, with a coverage of 94% of the neonates.2 Briefly, the relative incidence of neonatal screening test results in this program is 56.3% with an HbFS profile (SS or Sβ0-thalassemia), 39.4% with an HbFSC profile (HbSC), 3.6% with an HbFSA profile (Hb Sβ+-thalassemia) and 0.7% with an HbFSD profile.15
All data on clinical, laboratory and imaging tests were extracted from medical records and registered in a special database using the Access software (Microsoft®). As stated in the Introduction, TCD at HBH was started in 2007 and, accordingly, children born from 1999 to 2004 had no TCD at the age of two, as recommended by the protocol set in 2007.
Of the 718 children screened in that period, 675 (94%) had the HbSS profile and 43 (6%) had the HbSβ0-thalassemia subtype; 375 (52.2%) were girls. Ten children (1.4%), among them five girls, with the HbSS subtype died. The mean age at death was 8.7 years and none of these deaths was due to stroke. Children living in other cities under clinical and outpatient follow-up in the other six blood centers in the state were not included in the study. Neither were children with HbS/hereditary persistence of fetal hemoglobin, HbSβ+-thalassemia and HbSC and HbSD genotypes. The diagnosis was based on neonatal screening tests by isoelectric focusing electrophoresis and high-resolution liquid chromatography performed by the NUPAD. After 2010, the diagnosis was confirmed by allele-specific PCR for codon seven of the HBB gene.
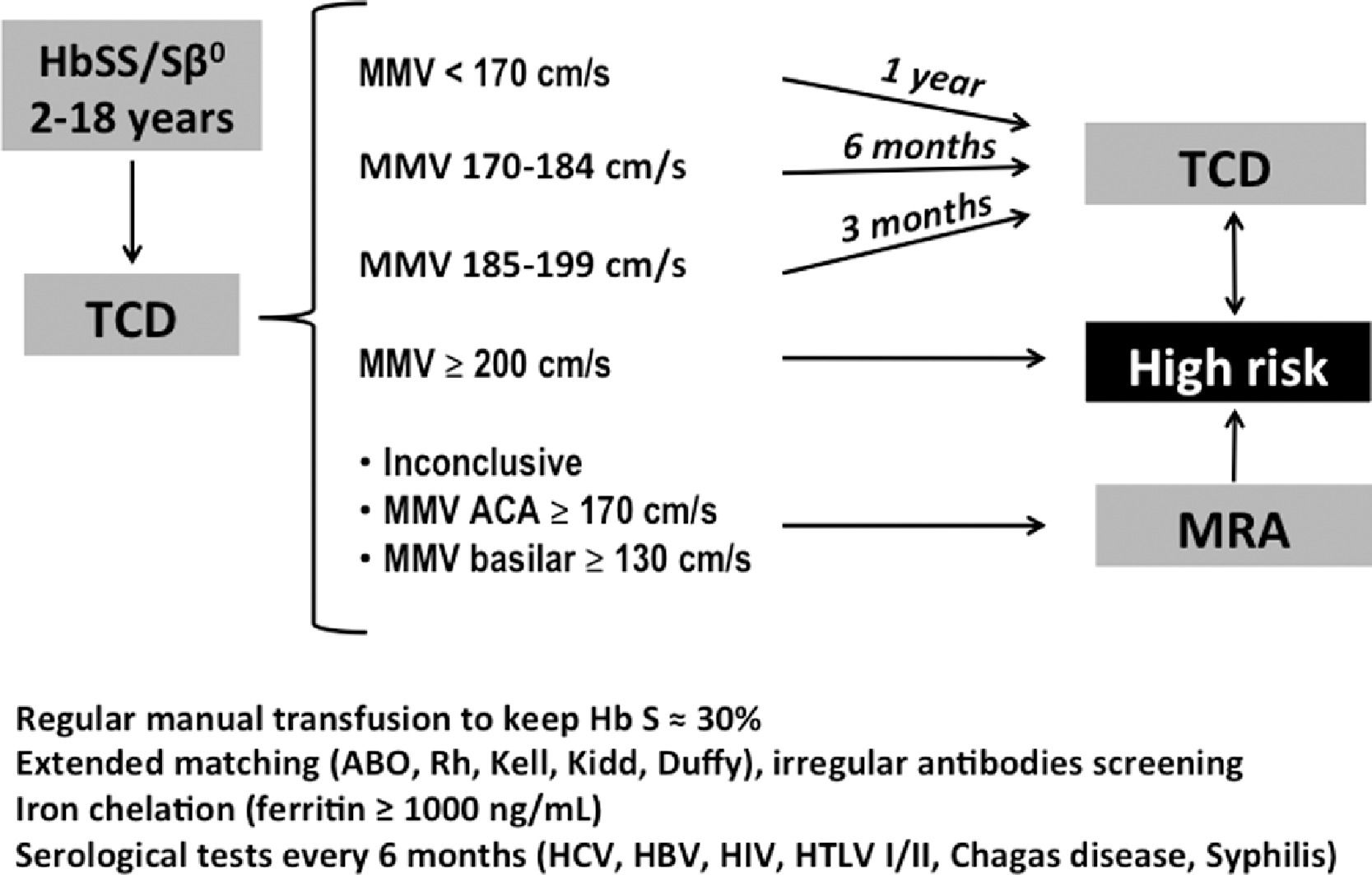
The TCD testing was performed or supervised by one of the authors (C.M.S.), trained at the Medical College of Georgia, USA, using the Stroke Prevention Trial in Sickle Cell Anemia (STOP) protocol with pulsed TCD and 2-MHz probe, and the complete Doppler test was performed with insonation of 15 arterial segments of the Willis polygon: M1 segment of the middle cerebral artery (MCA) and verification of all its extension every 2 mm, bifurcation of the internal carotid artery (ICA), distal or terminal internal carotid artery, anterior cerebral artery (ACA) and posterior cerebral artery (PCA) in both transtemporal windows and in the basilar artery in the transforaminal window. The maximum mean velocity (MMV) recorded in each artery was considered the most representative velocity. TCD results were classified by cerebral blood flow velocity in the MCA and ICA: 70 – 169 cm/s, low risk (“normal” TCD test); 170 – 184 cm/s, low conditional; 185 – 199 cm/s, high conditional, and; ≥ 200 cm/s, high-risk (“abnormal” TCD test).10
Absence of MCA insonation on one side, MMV < 70 cm/s in one of the MCAs or absence of transtemporal acoustic windows were considered inappropriate TCD tests, with an undefined risk for ischemic stroke.10 Tests classified as “abnormal” (high-risk) were repeated one or more times (confirmatory tests) at an interval of 2 to 4 weeks.
All the children in the high-risk group were referred to start the RTP. The regular intervals between TCD examinations were 12, 6 and 3 months for children with low risk, low conditional risk and high conditional risk, respectively. Children with inadequate or inconclusive results, with ACA MMV ≥ 170 cm/s or with basilar artery MMV ≥ 130 cm/s were referred to MRA testing. Those with signs of vasculopathy were also classified as high-risk for stroke and referred to the RTP (Figure 1).
This research was approved by the Ethics Committees of the Federal University of Minas Gerais (UFMG) and the Hemominas Foundation (Hematology and Hemotherapy Center of Minas Gerais). The informed consent form was presented to the legal guardian of the potential participant and the consent form, when necessary, was presented to the child himself or herself (children aged ≥ 7 years).
Statistical analysisThe data on all the children in the study are summarized as absolute and relative frequencies. From a previous smaller study performed by the same research group,5 39 children out of the 54 of the present study had an indication for primary prevention of stroke due to high-risk TCD or abnormal MRA. They had been genotyped for co-inheritance of the deletional -α3.7 thalassemia and had baseline hematological data recorded for that study. They were compared to the 369 SS children who had not undergone stroke or TIA as an event preceding the first TCD and who also had genetic and hematological data and belonged to the cohort of the present study. This was necessary for statistical nonbiased comparability. These two subgroups were compared for sex, age at the time of the first TCD and baseline hematological variables. Sex and alpha thalassemia co-heritage were compared in a simple 2 × 2 table and subjected to the Fisher's exact test. The other quantitative variables had the means of the two subgroups compared using the Student's t-test, considering the distribution of values as heteroscedastic, i.e., with significantly different variances. An alpha error probability lower than p < 0.05 was considered statistically significant.
ResultsThe 718 SCD children who underwent a follow-up at the HBH underwent at least one TCD. Fifty-four children (7.5%), all homozygous for the βS gene, were considered at high-risk for stroke: 45 children (83.4%) for exhibiting high-risk TCD and nine (16.6%) for presenting MRA findings with signs of cerebral vasculopathy after inadequate or inconclusive TCD; 36 (66.7%) were girls.
The mean age of the 54 children at the first TCD was 4.9 years. The mean age of the 45 children with high-risk TCD was 5.2 years and the mean age of the nine children diagnosed with severe vascular injury by MRA at the first TCD was 4.5 years. At the end of the cohort follow-up, in December 2018, the mean age of the 54 children was 13.3 years.
Of the 54 children, 51 initiated the RTP at a mean age of 6.3 years. The parents of three children refused the transfusion program. Two had been screened as high-risk by TCD. One of them had a stroke 3 months after the high-risk TCD and a transfusion regimen for the secondary prevention of a new event was started. The second child started HU therapy and presented reversal to low-risk 3 years later without a stroke. The third case presented MRA changes in 2014. She had already begun being treated with HU since 2012 due to abnormal TCDs in four successive examinations. Her parents also refused to start regular transfusions. She did not have a stroke in the clinical follow-up; thus, she continued on HU therapy.
Of the 43 children with high-risk TCD who started RTP after the confirmatory examination, 29 (67.4%) reverted to low-risk. Of these, the 18 (62.1%) who had undergone at least one year of the RTP with no signs of vasculopathy or SI evidenced by imaging examinations (MRA + MRI) were started on HU at the maximum tolerated dose (MTD), if not already using it for other clinical indications. The transfusion program was maintained for another 6 months and then discontinued. None of them had a subsequent stroke. Nine children (31%) remained in the RTP associated with HU and two (6.9%), only in RTP because the parents refused HU (Table 1). Stroke was not observed in any of these 29 patients.
Follow-up of 45 children with high-risk TCD and primary prevention of ischemic stroke in a cohort of newborns in Belo Horizonte, Brazil.
TCD: Transcranial Doppler; RTP: regular transfusion program; HU: hydroxyurea; BMT: bone marrow transplantation.
Six children in the RTP (14%) presented only conditional-risk reversion in subsequent examinations, even if HU was associated with the RTP. One of them underwent bone marrow transplantation and was cured. None of the six children had a stroke over the clinical follow-up.
Eight children (18.6%) continued presenting high-risk TCD, even after RTP associated with HU. Two of them had a stroke, at 6 and 1.5 years after the initial high-risk TCD, respectively; they were retained in the RTP for secondary prevention of new strokes (Table 1).
All the eight children being started on the RTP due to significant MRA abnormalities (Table 2, Figure 2) concomitantly used HU. The RTP was discontinued in two patients, one due to alloimmunization, with no compatible blood regularly available, and the other because a new MRA 1.5 years after the first showed abnormality reversal and three consecutive TCD studies showed a low risk for stroke. Both patients were treated with HU. None of the eight children had a stroke over the clinical follow-up.
Children submitted to the primary prevention program after inconclusive transcranial Doppler and diagnosis of being at high risk for ischemic stroke by abnormal magnetic resonance angiography (MRA) images.
| Children | Age at 1st TCD (years) | Abnormalities in MRA or MRI |
|---|---|---|
| #1 | 3.7 | 07/2016 - MRA: partial occlusion of the proximal segment M1 of the left middle cerebral artery. 06/2018 - MRA: no abnormality. 07/2016- MRI: no abnormality |
| #2 | 3.0 | 09/2013 - MRA: stenosis of the left internal carotid artery; vascular proliferation type moyamoya. 07/2015 - MRA: stenosis of the right internal carotid artery and moyamoya syndrome |
| #3 | 3.4 | 10/2018 - MRA: stenosis of both internal carotid artery, anterior cerebral arteries and middle cerebral arteries; moyamoya syndrome. MRI: gliosis and silent infarcts |
| #4 | 7.5 | 05/2015 - MRA: moyamoya syndrome |
| #5 | 7.6 | 04/2012 - MRA: stenosis of left internal carotid and carotid bifurcation |
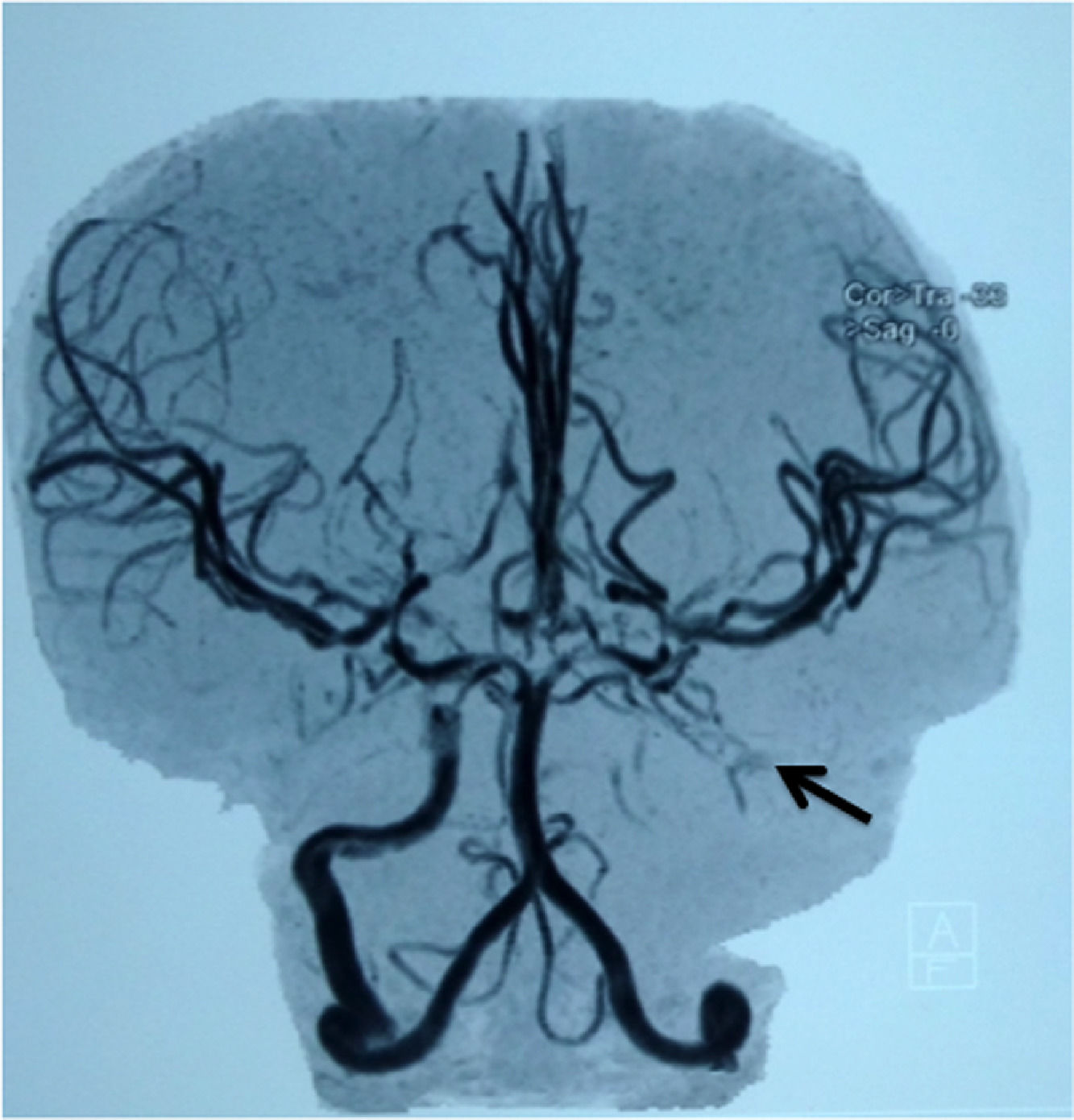
| #6 | 4.3 | 08/2013 - MRA: severe stenosis of the right internal carotid artery and occlusion of the left internal carotid artery. 10/2015: MRA: occlusion of the internal carotid arteries. 09/2018: MRA: severe vasculopathy |
| #7 | 2.6 | 05/2017 - MRA: stenosis of both internal carotid arteries and moyamoya syndrome. MRA - 02/2019: progression of vasculopathy; probable silent infarct and moyamoya syndrome |
| #8 | 4.4 | 02/2009 - MRA: stenosis of both middle cerebral arteries + moyamoya syndrome. Areas of left frontoparietal cerebral softening, probably related to prior stroke |
| #9* | 3.8 | 03/2014 - MRA: stenosis of both middle cerebral arteries + moyamoya syndrome |
Magnetic resonance angiography in the child #6 (Table 2), showing occlusion of the left internal carotid artery (arrow).
As mentioned in the statistical analysis, 39 of the 54 children (72.2%) at high risk for stroke were compared with 330 SS children. These 330 children did not have a stroke or TIA before the first examination and had no high-risk TCD over their clinical follow-up. Of the 39 in the RTP, only three were due to vascular lesion signs indicative of high risk for stroke at imaging examinations; the others initiated RTP because of a high-risk TCD.
There was a significant predominance of girls (66.6%) in the 39 children who started the RTP, compared to the others (48.2%; p = 0.04). The age at the first TCD was not significantly different in both groups (p = 0.4). Alpha-thalassemia co-inheritance was present in only 12.8% (5/39) of the children who started the RTP. In the other group, co-inheritance was present in 30% (99/330, p = 0.024, Table 3).
Demographic and hematologic characteristics of 39 children with SS disease on a regular transfusion program for ischemic stroke prevention, compared to 330 children for whom the transfusion program was not indicated[5].
| With primary stroke prevention (n = 39) | Without primary stroke prevention (n = 330) | p* | |
|---|---|---|---|
| Female | 26/39 (66.7%) | 159/330 (48.2%) | 0.04 |
| Mean age (years) at first DTC (SEM⁎⁎) | 5.37 (0.34) | 5.68 (0.13) | 0.40 |
| Basal Hb, g/dL (SEM) | 7.49 (0.11) | 7.93 (0.05) | 0.001 |
| Basal leukocyte count x109/L (SEM) | 16.44 (0.57) | 15.01 (0.19) | 0.031 |
| Basal reticulocyte percentage (SEM) | 18.11 (0.42) | 14.25 (0.22) | <0.00001 |
| Basal platelet count x109/L (SEM) | 430.76 (13.32) | 413.77 (6.12) | 0.25 |
| Porcentagem de Hb Fetal (SEM) | 13.10 (1.10) | 14.54 (0.14) | 0.23 |
| α-thalassemia co-inheritance | 5/39 (12.8%) | 99/330 (30.0%) | 0.024 |
The 39 children in the study presented lower mean total Hb and higher leukocyte and reticulocyte counts than the group of 330 children, all statistically significant comparisons. The mean platelet count and fetal hemoglobin concentration showed no significant differences (Table 3).
DiscussionStroke is one of the most serious complications of SCA. The increased brain blood flow velocity detected by TCD in the distal MCA and/or ICA is a risk factor for stroke in children and adolescents, with the RTP being effective in reducing the risk of the event. In the present study, the feasibility of identification of children at risk for stroke by TCD was demonstrated at the HBH; the RTP reduced the brain blood flow velocity in most patients detected as having a high risk for stroke (Table 1).
The STOP study screened 1930 children, identified 130 with abnormal TCD (MMV ≥ 200 cm/s) and showed a 92% reduced occurrence of stroke in the group that received regular transfusions, compared to the group under observational conduct. Twelve stroke events were recorded over the 16-month study, 11 in the conservative treatment group and only one in the transfusion group.10 However, transfusions were not free of complications and the time of primary prevention with chronic transfusions was questioned, which resulted in the STOP II study. After 30 months of transfusions, 41 patients had their transfusions discontinued, with 14 of them converting to high-risk by TCD and two of them had strokes, indicating the need to maintain regular transfusions.16 In 2006, a new evaluation of the STOP study showed that six more strokes occurred in addition to the 12 stroke events over the study period, five of them in the transfusion group. The other occurred in a patient who left the transfusion regimen right after the end of the STOP study. After a 30-month follow-up, patients who had abnormal TCD flattened the survival curve, with reduced risk of stroke in 40% over 3 years, suggesting that a subgroup of patients decreased the risk of the event, even without transfusions.12
In France, 92 children from the Créteil neonatal cohort, identified with abnormal TCD and with a mean age of 1.7 ± 0.6 years at the first examination, underwent a follow-up. The TCD was normalized (MMV < 170 cm/s) in 83.5% (76/91) after 0.3 to 6.9 years of chronic transfusion. Fifteen patients maintained abnormal TCD, even with transfusion, and the five of them, who had compatible donors, underwent hematopoietic stem cell transplantation (HSCT). Forty-five patients had the transfusions discontinued and received HU after TCD normalization and another 24 children with compatible donors also underwent HSCT. No patient had a stroke after starting the chronic transfusions. Only one 1.5-year-old child had an event after the abnormal TCD and before starting the transfusion regimen.17
In the US, 88 children from the STOP II study were analyzed, with 46 (52.3%) presenting normalized TCD. The tests were still above or equal to 200 cm/s in 19 children (21.6%), although with significantly decreased velocity in several children. The velocity reverted to the level of conditional risk in 17 children (19.3%). At a maximum follow-up time of 3.5 years, no child had a stroke in any of the subgroups mentioned.18 Therefore, these data corroborate our study, except for the two cases of stroke at 6 and 1.5 years after the beginning of the RTP and subsequent addition of HU.
A recent Brazilian study reported the follow-up of 15 patients with abnormal TCD under chronic transfusion for primary prevention of stroke, with a progressively decreased MMV by TCD in 14 patients. After at least 2 years, 11 patients showed normal results in the test, three reverted to conditional-risk, and only one maintained an abnormal result. None of the patients had strokes. The iron overload approach was effective with oral chelator and only one patient had alloimmunization.19
In this study, in the 18 children who had normalized TCD and no signs of vasculopathy or parenchymal brain injury, RTP was replaced by HU at MTD after one year. Before the publication of the TCD With Transfusions Changing to Hydroxyurea (TWiTCH)19 study, French researchers already used the replacement of chronic transfusions by HU or HSCT as a stroke prevention protocol for patients with compatible donors after TCD normalization.3 Subsequently, the TWiTCH study demonstrated the non-inferiority of HU in the primary prevention of stroke after at least one year of regular transfusion regimen in patients not presenting with vasculopathy signs or brain tissue lesions on imaging examination.20
This study reported that six patients reverted to conditional-risk by TCD, and none of them had a stroke over the follow-up period. As already mentioned, one of these children with alloimmunization underwent a bone marrow transplant in 2010, and is presently well and cured. In 2011, the TCD was normal. The literature reports that in the presence of severe SCA phenotypes with complications, absence of HU response, and availability of compatible donor, the patient should undergo transplantation.21
The comparison between our results with those of the French group showed inferior results in the present cohort. This difference could be explained by the later screening of the patients, with a mean age of 4.8 years at the first TCD, compared with that of 1.7 ± 0.6 years in the French study. The highest incidence of ischemic stroke is in the age range of 2 to 5 years, and cerebrovascular disease is progressive and irreversible in the most severe cases even with chronic transfusions. The lower adherence and more limited resources available in the public health system in Brazil should also be highlighted.
The French cohort reported that MRA scans performed 3 to 6 months after the abnormal TCD in 91 patients showed that 65 (71.4%) of them presented normal exams, and 26 (28.6%) showed signs of vascular stenosis. The MMV shown by the TCD was significantly higher in patients with vascular stenosis. Of the patients with normalized TCD, seven presented vascular stenosis by MRA, which receded during transfusion therapy.17 In this study, nine children underwent RTP based on the MRA findings since TCD was inconclusive.
In the present study, the most severe complication of the transfusion regimen was alloimmunization, which was present in 27% of children already in 2013, with antibodies mainly against the Rh system (anti-C and anti-E antibodies) and the Kell22 system. In 2015, the alloimmunization rate in the cohort was already 45% (data not shown). Although the HBH has performed extended erythrocyte phenotyping since 2004 and genotyping since 2006, this alloimmunization rate is quite high and makes it difficult to find compatible erythrocyte concentrates to maintain regular transfusions for patients with rarer phenotypes. Alloimmunization was reported in 58% of patients who were chronically transfused at a hospital in Philadelphia, with erythrocyte genotyping being suggested as a complement to serologic tests. This technique could refine erythrocyte compatibilization, reducing alloimmunization. However, there are still questions about the real effectiveness of this technique in reducing the problem, and additional studies are needed to recommend this procedure.23-25
It is currently not possible to identify predictive factors of SCA severity right at birth, but it is possible to outline a severity profile in the first 2 years of age by observing clinical and hematological progression that show several phenotypes in children with the same genotype.26-28 The hematological variables of the 39 children analyzed in the present study, which were part of the previous study sample of this cohort (369 children), were compared with those of children who did not have strokes and TIA or signs of cerebrovascular disease.5 As in the previous studies with the same cohort, the protective action of alpha-thalassemia co-heritage for cerebrovascular disease29 was corroborated, as also reported by others.30
In the present study, children with a high-risk TCD had a lower mean total basal Hb and higher leukocyte and reticulocyte count, similar to those in previous studies with a subsample of the present cohort.5 A review of the CSSCD data showed that the highest reticulocyte count in the first year of life, even in asymptomatic patients, had the highest clinical predictive value for stroke and mortality in children. Basal hemoglobin was also a significant factor, although at a lower intensity. Basal leukocytes and dactylitis, previously considered relevant, were not significant when adjusted for reticulocyte count.26
The limitations to this study include its retrospective design, the absence of RMA images for some children with high-risk TCD at the time RTP was started, and incomplete data on alloimmunization during or after RTP.
In conclusion, this study showed TCD as a feasible tool to screen risk for stroke, with prophylactic transfusions being effective in reducing brain blood flow MMV and preventing primary events. More than 60% of high-risk TCD patients reverted to low risk, and more than 80% reverted to low or conditional risk. Only two children progressed to stroke, even after using prophylactic transfusions and HU.
ConclusionAlloimmunization is a major problem in the management of children in the RTP; thus, new strategies to prevent and control cerebrovascular disease are expected to ensure a better quality of life for these patients.
The authors acknowledge all subjects and parents for their cooperation in the study. The authors also thank the Fundação Hemominas, Núcleo de Ações e Pesquisa em Apoio Diagnóstico (Nupad-UFMG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; award number 312112/2020-3) for their financial support.