Acute myeloid leukemia is a challenging disease, due to a poor prognosis in developing countries. Herein, we aim to describe the clinical characteristics and outcomes after chemotherapy and transplantation.
MethodsA retrospective analytic observational study was performed with patients under 18 years of age with newly diagnosed acute myeloid leukemia treated at a referral center in Colombia. Two groups were compared: induction therapy (IT) and induction therapy plus consolidation (IT + C). The survival analysis was performed using the Kaplan–Meier method.
ResultsWe analyzed 34 patients diagnosed with acute myeloid leukemia; 20 received hematopoietic stem cell transplantation. Most were French-American-British (FAB) classification types M1, M5 and M0. The transplantation was haploidentical in 65%, conditioning was myeloablative in 67% and graft-versus-host disease prophylaxis was performed with post-transplant cyclophosphamide in 70%. Overall, the 5-year survival was 52% and the overall 5-year survival in the transplanted group was 80%. There were 16 deaths; in the IT group, n = 12, and in the IT + C group, n = 4. In the former, the main cause of death was septic shock and in the latter, it was relapse.
ConclusionTransplantation is a safe option. Receiving treatment and supportive measures in hematopoietic stem cell transplantation units is necessary to avoid infections, especially during induction cycles.
Acute myeloid leukemia (AML) represents a heterogeneous group of clonal diseases in the hematopoietic tissue. Each subtype has different cytogenetic, molecular genetic features and antigenic expressions related to clinical manifestations, therapies and prognosis.1 AML is classified according to the French-American-British classification (FAB)2 and the 2016 revised World Health Organization (WHO) criteria.3
The incidence of childhood AML is 5 to 7 cases/million, corresponding to 15 to 25% of all childhood acute leukemias.4 Latin America has an estimated incidence and mortality rates of 4.3 and 1.2 cases per 100,000 people, respectively.5 In Colombia, AML represents 5 to 6% of all childhood cancers;5. Reports from southwestern Colombia show that the 3-year survival is above 33%.6
In recent times, overall survival (OS) rates greater than 70% after 5 years have been reported in high-income countries7; in low- and middle-income countries, the 4-year survival is reported in 47%.8 The best therapeutic results are achieved through improved diagnostic techniques, treament-monitored minimal residual disease (MRD) follow-up, intensification of the treatment, hematopoietic transplantation, better supportive care, rescue therapies and targeted nutritional interventions.7,9,10
The treatment of AML in pediatrics is performed with intense chemotherapy regimens based on anthracycline and cytarabine during induction, followed by consolidation therapies, as well as allogeneic hematopoietic stem cell transplantation (HSCT).4 When HSCT is performed, preparative or conditioning regimens include combinations with Fludarabine in most cases11,12; the addition of 4 Gy total body irradiation (TBI) showed a low non-relapse mortality rate, compared to those who received the conventional myeloablative conditioning regimen, in both adults and children.13 Related to donor type, unrelated umbilical cord blood and haploidentical transplantation are equally effective options for patients without human leukocyte antigen (HLA)-matched donors.14
The aim of this study was to characterize the pediatric population diagnosed with AML at a high complexity center in an upper-middle-income country15 to determine the primary clinical outcomes associated with treatment.
Materials and methodsThis protocol was approved by the institutional ethics committee (#1502), as per the principles established in the Council for International Organizations of Medical Sciences (CIOMS) guidelines and the Declaration of Helsinki.
We performed an analytical, observational, retrospective cohort study. Data were collected from the medical records of patients diagnosed with AML under 18 years of age treated at Fundación Valle del Lili (FVL) Teaching Hospital, a high-complexity referral center for HSCT and hematologic neoplasms management for the Colombian southwest.
All patients under 18 years of age who received a de novo diagnosis of AML between January 2011 and December 2020, seen at FVL, were included. Exclusion criteria included diagnoses of promyelocytic leukemia, leukemia associated with Down's syndrome, juvenile myelomonocytic leukemia, myelodysplastic syndrome, leukemia as a second neoplasm and patients with previous extra-institutional diagnosis and management.
DefinitionsThe AML treatment initially depends on the patient's risk classification. It consists of the induction stage using the "7 + 3" protocol, or 7 days of treatment with cytarabine (cytosine inhibitory analog) co-administered with 3 days of treatment with an anthracycline agent, followed by consolidation therapy, with chemotherapy or allogeneic HSCT. The latter contemplated high-risk AML patients in their first complete remission, with MRD more significant than 0.1%, at the end of the first or second induction cycles.4
For donor selection, the matched sibling donor was the primary option. If not available, the best related haploidentical donor (Haplo) was selected according to the following: the absence of recipient HLA antibodies against donor antigens, male sex, younger age, matched cytomegalovirus (CMV) serostatus, matched ABO group and, for female donors, the lowest number of prior pregnancies.16 A matched unrelated donor registry search was not readily available.
The conditioning regimen was defined according to the donor type and classified as myeloablative and non-myeloablative.17 We use Fludarabine-based conditioning regimens.11,12 Patients with MRD < 0.1% received a conditioning regimen with Busulfan + Fludarabine + low dose total body irradiation (400 cGy)13,18 Those with MRD > 0.1% received cytoreductive treatment combined with reduced-intensity conditioning and HSCT.19,20 The graft vs. host disease (GvHD) prophylaxis was assigned according to the donor type; in the case of haploidentical donors, PTCy and, in some cases, anti-thymocyte globulin (ATG) was used11,21;- In identical donors, it was based on the calcineurin inhibitor and methotrexate (MTX).22 All patients received antibiotic, antiviral and antifungal prophylaxis.
The nutritional status, based on the World Health Organization (WHO) parameters of 2006/2007, was also assessed. The classification was made by z scores, considering sex, age, weight and height; the weight/age was used for children under five and the BMI/age, for children aged five and over. Patients were classified as underweight or non-underweight, based on a Z score < -2 or ≥ -2, respectively.23
The overall survival was defined as the time from diagnosis to death from any cause, or the last recorded follow-up. The event-free survival was defined as the period from diagnosis to relapse or death. The post-transplant cause of death was taken as any cause of death cause following transplantation.24
Statistical analysisA descriptive analysis was performed for all variables. Categorical variables were summarized as proportions and quantitative variables were expressed as medians, with their interquartile ranges (IQRs). Patients were classified into two groups, based on whether or not they received consolidation therapy, the IT group being those without consolidation therapy and the IT + C group, those with consolidation therapy. An additional analysis according to the year of diagnosis was considered in two periods: 2011 to 2016 and 2017 to 2020, to control for demographic characteristics related to the consolidation therapy used. Comparisons between groups of interest were made with the chi-square test for categorical variables and the Mann-Whitney U test for quantitative variables, according to the distribution of the variable evaluated by the Shapiro–Wilk test. A subgroup analysis of interest was performed in transplant recipients (pre-transplant MRD < 0.1 and ≥ 0.1). The survival calculation was performed by the Kaplan–Meier method and the two groups were compared according to the time frames previously stated. The comparison of the groups, according to the defined exposures, was performed with the log-rank test. For survival analysis, patients were right-censored at the time of death or the last follow-up before December 31, 2021. Clinical endpoints of the OS were recorded at 5 years from the AML diagnosis, and the OS and event-free survival (EFS) were recorded for transplant patients at 5 years from the HSCT. The Cox regression was used to identify the risk factors, considering the time to death before consolidation therapy. The variables analyzed were: the central nervous system compromise, initial leukocytes, FAB classification, Z score and antimicrobial prophylaxis, before or during the induction chemotherapy.
ResultsOverall groupBetween 2011 and 2020, there were 34 patients with de novo AML. The median age at the start of chemotherapy was 7.7 years (IQR 1.6 - 13). Males comprised 56%. Nine percent (n = 3) of patients were underweight. At diagnosis, 8.8% (n = 3) had central nervous system (CNS) compromise and 47% (n = 16) had initial leukocytes > 50,000. The most frequent FAB classifications in the group were M1 (26%), M5 (21%) and M0 (18%), the latter present only in the IT + C group. The karyotype information was found in 74% (n = 25) of the sample; 12% (n = 4) had abnormalities. Molecular tests were performed in 68% (n = 23), of whom 13% (n = 3) had abnormalities, and the FISH test was performed in 12% (n = 4), finding anomalies in 25% (n = 1). Sixty-five percent (n = 22) received consolidation therapy, 59% (20) with HSCT, and 6% (2) with chemotherapy. At the end of cycle 1, 58% (n = 18) had CR, increasing to 87.3% (n = 28) at the end of cycle 2. Patients with MRD < 1% in cycle 1 were 28% (n = 9) and, at the end of cycle 2, 53% (n = 17).
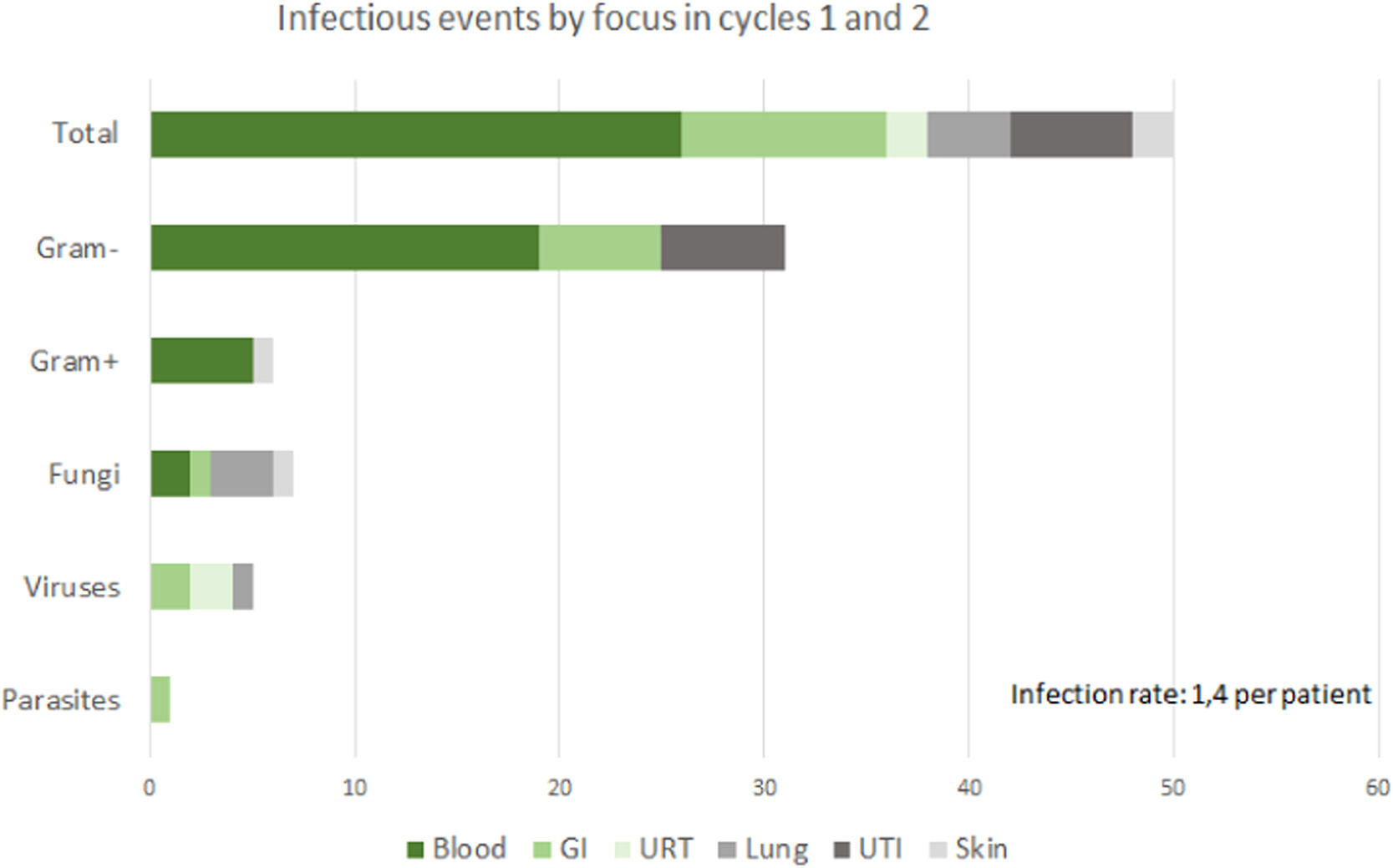
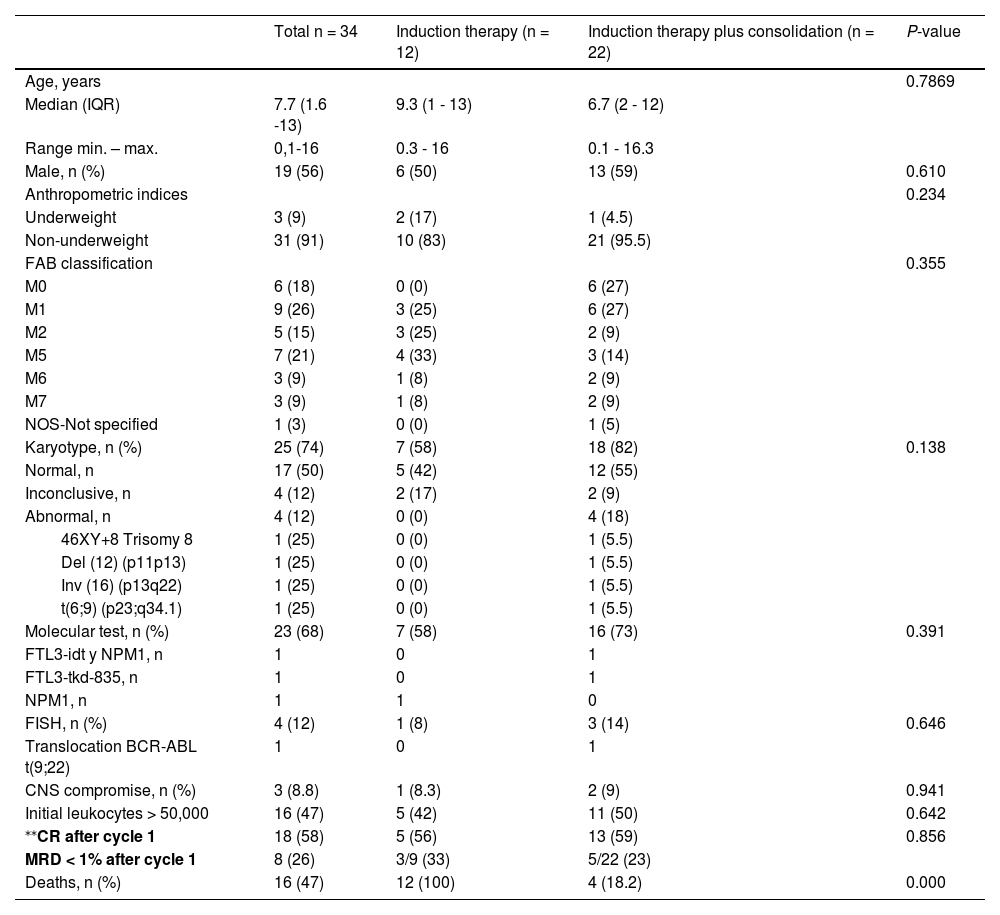
Of the 34 patients, seventy-six percent (n = 26) presented infectious complications during cycles one or two of induction chemotherapy; there were 50 infectious events. Of these, 31 (62%) were caused by gram-negative bacteria, 6 (12%) by gram-positive bacteria, 7 (14%) by fungi, 5 (10%) by viruses and 1 (2%) by a parasite. (Figure 1). Forty-four percent (n = 15) required total parenteral nutrition due to gastrointestinal dysfunction related to mucositis or acute GvHD. Seventy-six percent (n = 26) required admission to the Pediatric Intensive Care Unit (PICU). There were 16 deaths (47%); 71% (n = 12) of the deaths happened during the first and second induction cycles with chemotherapy; the causes of death were septic shock (n = 7), multiorgan failure (n = 4) and fungal infection (n = 1) and 29% (n = 4) died during consolidation with HSCT. Table 1 shows the differences between patients who did not receive consolidation therapy and those who did.
Sociodemographic and clinical characteristics.
| Total n = 34 | Induction therapy (n = 12) | Induction therapy plus consolidation (n = 22) | P-value | |
|---|---|---|---|---|
| Age, years | 0.7869 | |||
| Median (IQR) | 7.7 (1.6 -13) | 9.3 (1 - 13) | 6.7 (2 - 12) | |
| Range min. – max. | 0,1-16 | 0.3 - 16 | 0.1 - 16.3 | |
| Male, n (%) | 19 (56) | 6 (50) | 13 (59) | 0.610 |
| Anthropometric indices | 0.234 | |||
| Underweight | 3 (9) | 2 (17) | 1 (4.5) | |
| Non-underweight | 31 (91) | 10 (83) | 21 (95.5) | |
| FAB classification | 0.355 | |||
| M0 | 6 (18) | 0 (0) | 6 (27) | |
| M1 | 9 (26) | 3 (25) | 6 (27) | |
| M2 | 5 (15) | 3 (25) | 2 (9) | |
| M5 | 7 (21) | 4 (33) | 3 (14) | |
| M6 | 3 (9) | 1 (8) | 2 (9) | |
| M7 | 3 (9) | 1 (8) | 2 (9) | |
| NOS-Not specified | 1 (3) | 0 (0) | 1 (5) | |
| Karyotype, n (%) | 25 (74) | 7 (58) | 18 (82) | 0.138 |
| Normal, n | 17 (50) | 5 (42) | 12 (55) | |
| Inconclusive, n | 4 (12) | 2 (17) | 2 (9) | |
| Abnormal, n | 4 (12) | 0 (0) | 4 (18) | |
| 46XY+8 Trisomy 8 | 1 (25) | 0 (0) | 1 (5.5) | |
| Del (12) (p11p13) | 1 (25) | 0 (0) | 1 (5.5) | |
| Inv (16) (p13q22) | 1 (25) | 0 (0) | 1 (5.5) | |
| t(6;9) (p23;q34.1) | 1 (25) | 0 (0) | 1 (5.5) | |
| Molecular test, n (%) | 23 (68) | 7 (58) | 16 (73) | 0.391 |
| FTL3-idt y NPM1, n | 1 | 0 | 1 | |
| FTL3-tkd-835, n | 1 | 0 | 1 | |
| NPM1, n | 1 | 1 | 0 | |
| FISH, n (%) | 4 (12) | 1 (8) | 3 (14) | 0.646 |
| Translocation BCR-ABL t(9;22) | 1 | 0 | 1 | |
| CNS compromise, n (%) | 3 (8.8) | 1 (8.3) | 2 (9) | 0.941 |
| Initial leukocytes > 50,000 | 16 (47) | 5 (42) | 11 (50) | 0.642 |
| ⁎⁎CR after cycle 1 | 18 (58) | 5 (56) | 13 (59) | 0.856 |
| MRD < 1% after cycle 1 | 8 (26) | 3/9 (33) | 5/22 (23) | |
| Deaths, n (%) | 16 (47) | 12 (100) | 4 (18.2) | 0.000 |
A total of 20 patients received transplants, 10 (50%) had a pre-HSCT MRD < 0.1% and 10 (50%) had an MRD ≥ 0.1%. Thirty-five percent (n = 7) of transplant recipients received two cycles of chemotherapy, 45% (n = 9) received three cycles and 20% (n = 4) received four cycles. Sixty-five percent (n = 13) had haploidentical transplantation, and 35% were identical. The cell source was bone marrow in 55% (n = 11) and peripheral blood in 45% (n = 9). Conditioning was myeloablative in 70% (n = 14) of the cases and nonmyeloablative in 30% (n = 6). Sixty percent (n = 12) received total body irradiation (TBI). Twenty percent (n = 4) received anti-thymocyte globulin (ATG). The GvHD prophylaxis was based on PTCy-based prophylaxis in 70% (n = 14) and cyclosporine-based prophylaxis in the remaining 30% (n = 6). There were four post-transplant deaths, of which one was due to relapse; three were transplant-related, one of which was due to septic shock, one was related to GvHD and one was related to multiorgan failure.
In this study, only the first transplant was considered. However, one death occurred after the second transplant.
Risk factors for death before consolidation therapyRisk factors for pre-consolidation therapy death were evaluated, however, no parameter was statistically significant. Patients with FAB M0 did not die, nor did those who received more than two cycles during the chemotherapy period.
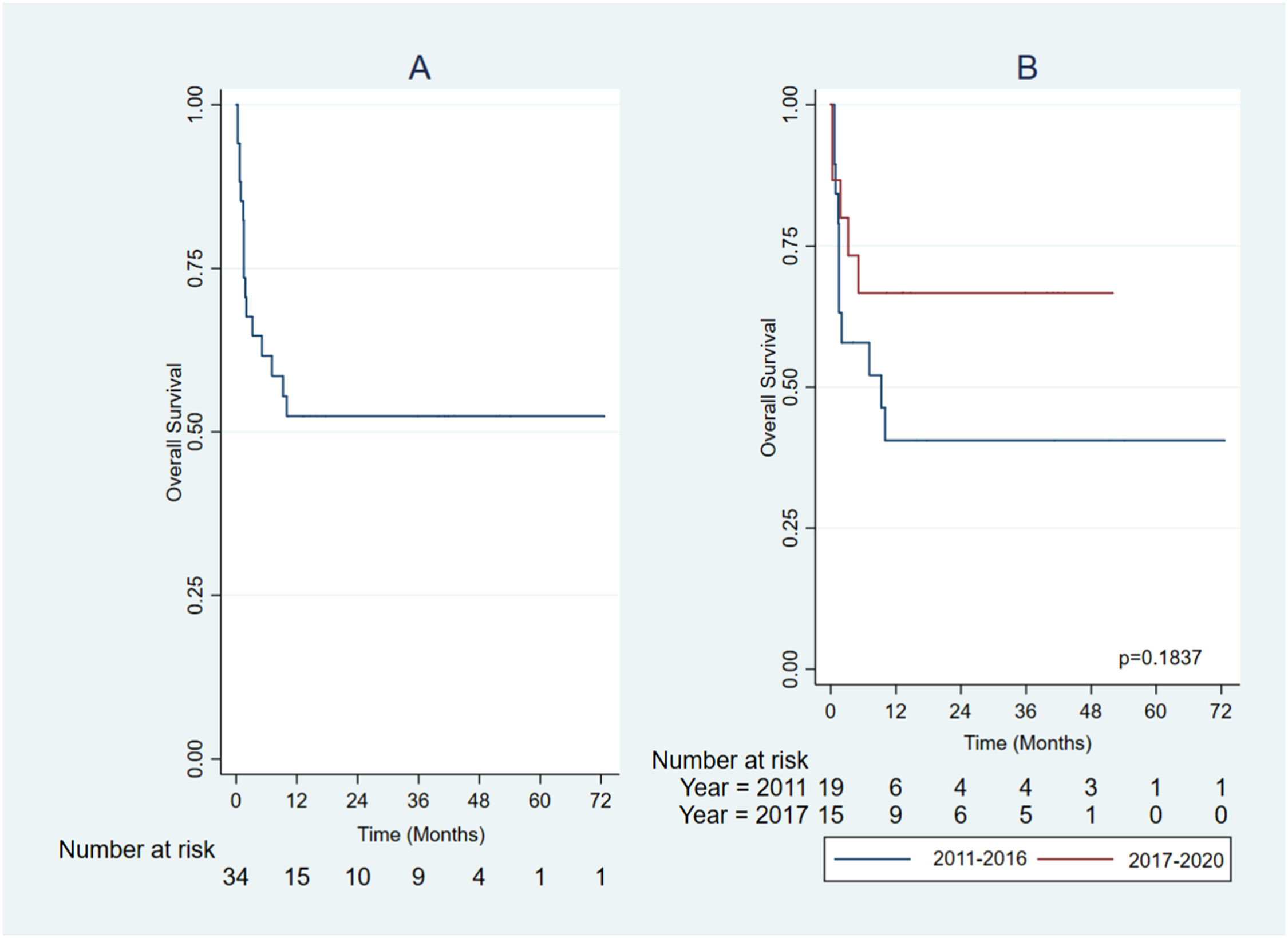
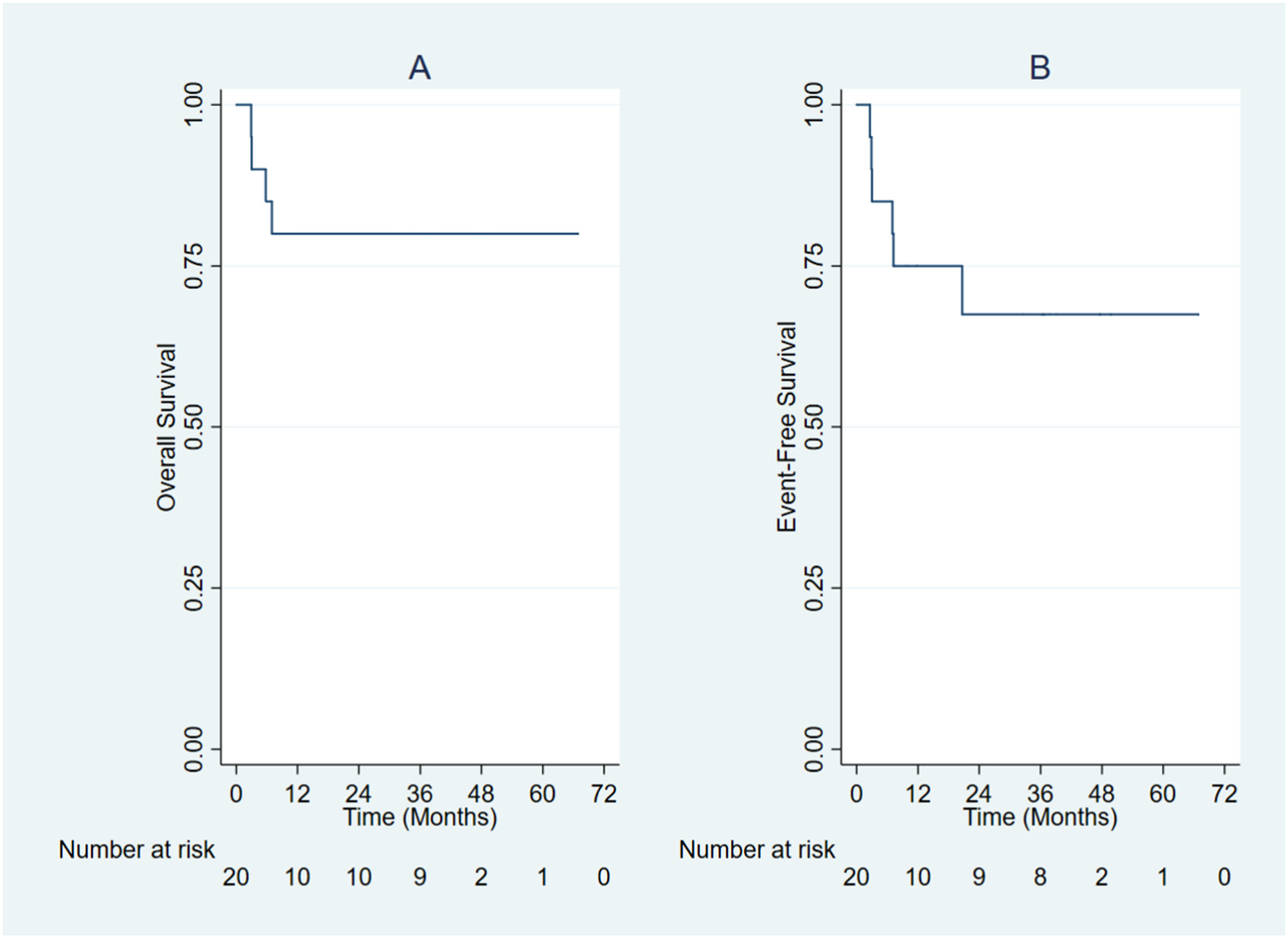
SurvivalThe overall 5-year survival for the entire group was 52% (Figure 2A). From 2011 to 2017, the overall 5-year survival was 40% and, between 2017 and 2020, it was 67%, which was not statistically significant (Figure 2B). The median follow-up for the total group was 10 months, ICR 1.5 - 40 (min. 0.3 – max. 72.6). In the group of patients who survived (n = 18) the median follow-up was 37.9 months, ICR 13 - 43 (min. 4 – max. 73). The overall 5-year survival in the transplanted group was 80% (Figure 3A), and the event-free survival in transplant recipients was 75% at one year and 67.5% at five years (Figure 3B).
In this study of 34 patients with AML in Colombia, we described the characteristics and outcomes of children who received treatment at a referral center. The overall 5-year survival in the present study was 49%, similar to another local study, with 53.5%, in patients receiving only chemotherapy.25 Most of our deaths (70.6%) occurred before or during induction, similar to the study by Ghafoor et al. 7.
Although high-income countries have shown improvement in treatment-related mortality over the last few years, this is not the case in upper-middle- or low-income countries. Despite the use of antimicrobials, approximately 45% of treatment-related mortality was due to an infection, compared to high-income countries, where it was between 5% and 11%.26 As infectious events remain high, strict measures of biosecurity and support are necessary to reduce mortality.27
In the present study, 72% of patients had an MRD ≥ 1% after the first induction, which induces a high chance of relapse and death27; therefore, our population has a high possibility of poor response to treatment, compared to other studies.9
In transplanted patients, there was no difference in having positive or negative MRD; this is quite different from what has been reported in the literature, where children with pre-transplant MRD-negative status have a better post-transplant prognosis than those with residual MRD (4). This may be associated with the chimerism-based donor lymphocyte infusion (DLI) strategy.
In our study, 14% died in the first six weeks, which is higher than that in high-income countries,27 but similar to that in upper-middle-income countries.28
In this study, transplanted and non-transplanted patients were comparable at diagnosis and we found a proportion of patients with white blood cell counts > 50,000, similar to those in reports in other developing countries.29 Therefore, we considered that survival was probably unrelated to the characteristics at diagnosis, but could be increased by improving supportive care during induction chemotherapy, as documented in the literature.27
The 18-month survival in the group without HSCT was 64%, similar to the 60% at 2 years in Suarez-Mattos et al. The overall 5-year survival in our study was 49%, similar to those in developing countries.25 The overall 5-year survival in the transplanted group was 74%, higher than the 47% between 2008 and 2012 in Rodrigues et a al.8 Upper-middle- and low-income countries still have economic and sociocultural limitations,2,8 affecting patient access to diagnosis and treatment, such as molecular/cytogenetic evaluation and standardized protocols.8 Nonetheless, our survival rate is still lower than those in high-income countries, which have been reported at approximately 70 to 75%.10 These countries are using personalized medicine and genomic characterization of relapsed disease,30 molecularly targeted therapy and immunotherapy, or are developing risk algorithms to recommend HSCT in the first complete response.31,32 There seems to be an improvement in survival in recent years (2017 - 2020), probably related to better supportive care, such as increased surveillance and infection control through intensified azole antifungal prophylaxis, early dental treatment, surveillance for colonization by multidrug-resistant microorganisms and access to the PICU, as reported.2,8,25
For upper-middle-income countries, chemotherapy for AML based on nutritional status at diagnosis could be a factor that contributes to the impact on survival.7 Although our study showed that the majority of patients were at an adequate weight for age at the beginning of treatment, we consider that in addition to supportive measures, stratification with nutritional status should be evaluated for the use of effective chemotherapies at lower doses and with lower toxicities.33
In our study, 60% of the patients received transplantation because most of them were classified as high risk, similar to those in Lee et al.,34 who performed transplantation in 51% of the cases. The indications for transplantation are not uniform, varying over time and according to the protocols proposed by different groups. However, there is a consensus regarding performing an HSCT with the most appropriate and available donor in patients during the first remission for high-risk or low-risk groups after relapse.35
Considering the context of these patients, improving the outcomes requires more resources and installed capacity of the HSCT units8 to centralize management and provide integrated treatment with supportive care from admission, avoiding in-hospital bed transfers and the risk of nosocomial infection and death. In lower-middle income countries, successful supportive care should consider nutrition, catheter care, mucositis care, pain management, hydration, early prophylaxis, timely treatment of infectious diseases, early access to a PICU, management of febrile neutropenia and isolation.10
This study has some limitations, such as its retrospective design and limited data collection in medical history records and access to complementary studies. The sample size was small, which limits multivariate analysis. However, our study shows improvement in terms of increased survival over the period studied and the need to implement patient supportive measures under the intensity of treatment, which can be studied in future research.
ConclusionThis study shows that transplantation is a safe option for high-risk patients. More efforts should be made to reduce infectious complications and improve stratification studies to classify patients appropriately based on the low-dose chemotherapy regimen that could be effective, especially for malnourished patients, and improve supportive care during the first two cycles.
Ethical approvalThe study was approved by the Ethics Committee on Biomedical Research under code 1502.
Author contributionsAll authors contributed to the study purpose and design. Material preparation, critical revision and analysis were performed by AAF, VL, PF and DM. The first draft of the manuscript and data collection were performed by EB. Quality control and statistical analysis of the data were performed by EM and EB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript version to be submitted and agreed to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.